| Pages:
1
2 |
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
US Pyrex kitchenware not Borosillicate glass!
Had some Pyrex break and the way it shattered made me question the material but never looked into it until now. It looks like the type of glass is
dependent upon region. Not too good IMO.
The following can be found on this page: https://en.wikipedia.org/wiki/Borosilicate_glass
| Quote: |
The European manufacturer of Pyrex, Arc International, uses borosilicate glass in its Pyrex glass kitchen products;[1] however, the U.S. manufacturer
of Pyrex kitchenware uses tempered soda-lime glass.[2] Thus Pyrex can refer to either soda-lime glass or borosilicate glass when discussing kitchen
glassware, while Pyrex, Bomex, Duran, TGI and Simax all refer to borosilicate glass when discussing laboratory glassware. The real difference is the
trademark and the company that owns the Pyrex name. The original Corning ware made of borosilicate glass was trademarked in capital letters (PYREX).
When the kitchenware division was sold, the trademark was changed to lowercase (pyrex) and switched to low thermal-expansion soda-lime glass.
|
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Yes, I think it has not been borosilicate for quite some time now. Over in Europe Pyrex brand kitchenware is still boro glass IIRC (edit: I see that's
in the quote now). Kitchen glassware in general has rather thick glass, which is undesirable because it increases the chance of cracking while
heating, even if it does increase durability.
[Edited on 2/5/2017 by Geocachmaster]
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Yes, we know.
The part that I didn't know was that Corning sold off their kitchenware division. Man, these guys sell everything.
In india, they established a chemistry glassware company and then sold it off. It is now under the name of Bomex.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Well I'm glad that other know, whether some or most, IDK, but it does seem a little odd that it differs from the US to EU, especially if someone reads
that Pyrex is Boro somewhere but doesn't mention that it can vary with intended use and region of distribution.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, those bastards took advantage of the fact that "Pyrex" is their trademark and not actually synonymous with "borosilicate glass". So they can
label anything they want to as "Pyrex" and there's nothing we can do about it, except learn to recognize borosilicate glass when we see it. (hint: if
it's thick and has a green tint, don't use it for chemistry!)
edit: I wonder if there's grounds for a class-action lawsuit, alleging that they knowingly misled people by doing this? LOTS of people, even
non-chemists have had glass cookware explode on them because they thought that "Pyrex" meant "borosilicate".
[Edited on 2/7/17 by Melgar]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I have a large collection of Pyrex pans and dishes that I use for chem all the time. Just because you can't heat it directly doesn't make them less
useful.
I use the large Pyrex mixing bowls as ice baths or warm water baths with an immersion heater. I fill one with xylenes to soak other glassware in for
removing sulfur, since xylenes don't play nice with plastics. They're great for crashing large, acidic solutions into cold water (like nitrations) and
you can get a 2-liter bowl at the goodwill store for $3, as opposed to a 2-liter boro beaker which is like $30.
Pyrex pans and baking dishes can be safely heated over a steam bath and in the oven, so they make great surfaces for drying wet crystals, evaporating
solutions, and regenerating desiccants. I do it all the time in my videos.
[Edited on 7-2-2017 by Praxichys]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Why, exactly, is Kitchen-ware being discussed on a Chemistry forum ?
Oh ! I see. If it's not politics any other garbage is fine ? OK.
Personally i like the french steel-teflon flan trays with detachable bottom plate for making small quiches. Perfect pro-looking result and instant
quiche-release.
Recently i got one with perforations, giving a really crispy texture to the pastry.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by Praxichys  | | I have a large collection of Pyrex pans and dishes that I use for chem all the time. Just because you can't heat it directly doesn't make them less
useful. |
I think what gets most people is the fact that you also can't cool it directly. Like, they'll set a hot Pyrex™ pan on a cold stovetop and have it
explode. And I agree that having semi-heat-resistant glass bowls and such is useful, it's just a bit disingenuous for them to allow it to be
labeled using a brand name that has become shorthand for "borosilicate glass". (Incidentally, since we use "pyrex" all the time here to mean that, I
guess we should capitalize it for the brand, and use lowercase to mean borosilicate? We need conventions!)
It'd be like having Kleenex-brand burlap, or Band-aid brand nicotine patches. Not useless so much as inconsiderate.
aga: Because a lot of people use glass cookware for chemistry. I used to have a Pyrex™ measuring cup that was graduated in mL and ounces, until it
exploded on me when heating it. Until then, I too, thought "Pyrex" meant borosilicate.
[Edited on 2/7/17 by Melgar]
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Doesn't soda lime glass also have a higher percentage of soluble glass besides not being resistant to thermal shock? While it's probably not a major
problem for non-analytic work, it's worth keeping in mind.
Batoussai.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I also use whatever i have that is suitable at the time.
Currently i got about quarter a kilo of sodium sulplate drying on the lid of a 20L paint bucket.
"Pyrex" has always just been a brand name, synonymous with kitchen glass that can go in an oven.
What it is composed of is pretty much irrelevant - it's not sold as chemistry apparati (which also shatter if treated badly).
My point is : why is so much said about trivial non-Chemistry nonsense these days ?
Someone does a Grignard and gets 3 or 4 replies.
Mention some kitchenware breaking and get how many ? Sheesh !
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by aga  | Someone does a Grignard and gets 3 or 4 replies.
Mention some kitchenware breaking and get how many ? Sheesh ! |
If you're trying to say something, this is a really weird battle to pick, considering improvised glassware is very much on-topic in this forum. Pyrex
brand kitchenware used to always be made out of borosilicate glass, and still is in many countries. Being able to discern the difference between
Pyrex-brand borosilicate cookware and Pyrex-brand tempered soda-lime cookware is fairly important if we need real borosilicate glass, improvised or
not, for a particular reaction or purpose.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=3384
http://www.sciencemadness.org/talk/viewthread.php?tid=12170
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The glassware used is definitely on-topic.
ONLY of the glassware is ever used.
(corningware is also good)
No evidence of any chemistry At All yet from the OP, so i dunno.
|
|
|
zwt
Hazard to Self
 
Posts: 84
Registered: 1-8-2016
Member Is Offline
Mood: No Mood
|
|
Here's some:
http://www.sciencemadness.org/talk/viewthread.php?tid=26378&...
http://www.sciencemadness.org/talk/viewthread.php?tid=61783&...
http://www.sciencemadness.org/talk/viewthread.php?tid=70072#...
Looks like attempts to prepare and purify reagents, the necessary first steps for those who can't just buy everything they need. You shouldn't have a
problem with that - you've also been complaining recently about people who don't prepare their own reagents.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by aga  | The glassware used is definitely on-topic.
ONLY of the glassware is ever used.
(corningware is also good)
No evidence of any chemistry At All yet from the OP, so i dunno. |
boi how many of your posts even contain chemistry? Over 6000 comments and probably somewhere just under 1% provide evidence that you've done any.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Amos  |
how many of your posts even contain chemistry? Over 6000 comments and probably somewhere just under 1% provide evidence that you've done any.
|
That is probably a reasonable question to aga but it is not a conversation that I think needs to happen in public. And the same goes for aga's
questioning of the chem content of other users' posts.
We all know that getting some good experimental chemistry in and sharing the results is the most satisfying thing about this board. But there are
many reasons why it does not always happen. Life has a way of getting in the way for all of us. I come here to think chem, read chem and discuss
chem even when I cannot do chem. I think that is ok.
Back on topic, I do have among my lab equipment a range of repurposed kitchenware items. I started collecting those before I got into buying proper
glassware. Although they are often useful and frequently cheaper, I do not trust the glass items to resist any kind of thermal shock or high
temperatures. Maybe I am being overly cautious. But I have seen exploding kitchenware before and it just aint pretty.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Agreed but I have my limits.
The only piece of kitchenware I stock at the moment is a big casserole dish I use for crystallization, which often entails pouring a liter of boiling
liquid directly into it. I often put it directly on a hot plate to evaporate solutions, too, just being careful to keep the temperature low and ramp
it up slowly. Hasn't caused any problems in about 2 years, so it can't all be that awful.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've use soda lime cookingware for a few lab tasks, generally trying to limit its exposure to temperature extremes that it would encounter in normal
use, and I haven't run into any problems. That stated, I wouldn't try heating it with a blowtorch or putting it over a Bunsen burner.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
worth remembering ... Otto Schott started selling DURAN borosilicate glass in 1893,
anything before that was soda-lime glass ...
i.e. Alchemy, the chemistry renaisance and all pre-20th century chemistry 
My borosilicate glassware with ground glass joints would have given Alchemists wet dreams 
https://en.wikipedia.org/wiki/Borosilicate_glass
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Just out of curiosity, I'm wondering about coffee pots like the ones for coffee machines. I've seen a lot of people use them on a burner (usually
gradually heating) as well as over a mild flame like an alcohol lamp. The glass is very thin compared to the Pyrex measuring cups and other Pyrex
"cookware" like small 1c storage dishes (great for evap containers for non-corrosive materials).
The glass on these coffee pots is very similar in thickness and color/transparency to the Pyrex beakers I have as well as Pyrex flasks, test tubes,
petri dishes, etc. They also break similarly as well with being dropped or hit with something heavy.
I can't imagine these are soda-lime but more like some boro type. I did heat NaHCO3 to not temp (350F -400) and them placed on a cold metal stove to
see if it could crack and it seemed fine. It was an older Mr Coffee purchased at a thrift shop in the US. I can't speak to other pots that may seem
similar but of "cheaper" brand or possibly construction.
Oh and for note, the REASON I brought this topic up is I had read in a post, on this board, that all pyrex was borosilicate and was indeed something
to look for - which is why they went to rubbage/garage sales and thrift at times because they found great uses for these when doing various
non-crucial experiments. Well I had an explosion experience with one and months later I posted this thread when I found the infor I linked to above.
AS always, some member (Cartman in his elder years - Respect my authoritah!!! and Homer Simpson hybrid) feels the need to make accusations and talk
smack when there may be many other people who saw the name post I did and could get hurt by this. Then it lead to personal attacks whe all he had to
do was a search, but his type just spews insults to anyone who Dares to not act in accordance to how HE/THEY believe is the proper and only way.
I thank all those who have mentioned something on my behalf and realize the glaring contradiction of 2 people, joined a couple months apart, one with
30x my posts (and a much MUCH higher non-chemistry post count than ANYONE on this forum) with a percent of member belligerence that is matched by NO
ONE else on the board - chasing newbies away for many unfounded reasons. This is what almost ANY mod and member would call a troll.
Again, I thank those who recognize that not all member have access to the means to perform complex reactions that require distillation kits or vacuum
filtration but by asking questions, especially those designed to highlight potential danger issues, is a magnitude more helpful that negativity spewed
by some.
[Edited on 10-2-2017 by RogueRose]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
There's this stuff.
https://www.youtube.com/watch?v=rc6EH2Gb90o
http://www.kavalier.cz/en/section/15-general-information.htm...
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Some cafetieres have nice 'beakers' at their core,
I broke a 2l one and I have a 600ml and an 800ml one, from charuty shops.
Some are PYREX, some are toughened glass, all can handle boiling water.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
My sister sent me a couple of these bottles out of the blue having mentioned I liked some thin-walled borosilicate food storage containers that looked
something like large petri dishes with high sides and lids. Happy little bottles I guess ...
http://1.bp.blogspot.com/-7IKum7Vkdoo/UYotsjH-b5I/AAAAAAAAAF...
https://static.fpv24.com/img/product/re/retblrp11gl47ly28/so...
http://www.retap.com/products/
http://www.retap.com/en/wp-content/uploads/2014/10/table-wit...
https://cdn4.thegrommet.com/media/catalog/product/cache/1/im...
https://cdn3.volusion.com/devfd.zdvnj/v/vspfiles/photos/rt1-...
 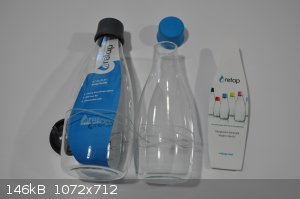 
[Edited on 9-9-2017 by Morgan]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by aga  |
Personally i like the french steel-teflon flan trays with detachable bottom plate for making small quiches. Perfect pro-looking result and instant
quiche-release.
Recently i got one with perforations, giving a really crispy texture to the pastry. |
A flan tray with perforations? Doesn't it leak badly when you melt the sugar?
I use ceramic ramekins mostly, sometimes a PYREX baking dish, but never a pyrex baking dish.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Sadly, much cookware in the US has been soda lime for years now. The index of refraction is different, but hard to measure trivially, and there are
several times of soda lime glass, so not trivial to tell them apart without a little work. But there are useful properties of both. The main
issue is that making borosilicate glass pollutes more than soda lime, thus making it in the US is nearly impossible now, just like doing any chemistry
is tough and expensive now.
|
|
|
| Pages:
1
2 |