| Pages:
1
2
3 |
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Benzaldehyde synthesis using aqueous nitric acid
Introduction
The procedure below describes the oxidation of benzyl alcohol to benzaldehyde in high yield using aqueous nitric acid as an oxidizer. Other methods of
oxidizing benzyl alcohol to benzaldehyde are well-known, including those using chlorochromates, persulfate, or activated manganese dioxide. The method
is advantageous in that it gives a high yield while using relatively simple equipment and more common, easy-to-obtain reagents than others.
Synthesis
50g (714mmol) of 90% nitric acid (homemade, concentration determined by titration, density 1.48g/mL) were placed in a 250mL round-bottom flask. The
nitric acid was cooled in a salted ice bath and a couple of mL of benzyl alcohol (technical grade, KYAntec) were added to the flask using a Pasteur
pipette.
 
The flask was manually swirled by hand to mix the reagents, and an immediate change in color from pale yellow to a bright yellow-green was noted. As
more benzyl alcohol was added, an upper layer separated and the color of this gradually deepened to an intense blue-green. The release of brown
nitrogen oxide vapors was also noted. The classic almond extract of aroma was already heavy upon the air between additions by this point.
Upon each further addition of benzyl alcohol and with stirring of the mixture, the color disappeared and became milky yellow, but after being allowed
to react, the blue color returned; the color change was used to follow the reaction, with each addition of benzyl alcohol occurring after the upper
layer had regained the unusual coloration. With some difficulty I managed to get enough light passing through the deep green mixture for a picture.
 
A total of 66g (610mmol) of benzyl alcohol were added over the course of about 4 hours. While an ice bath was used to regulate temperature at the
beginning of the experiment, as the reaction continues and the concentration of nitric acid falls, the reaction slows significantly and it is
sufficient to carry out the last third or so of the reaction at room temperature. Because a goal of this synthesis was to avoid benzyl alcohol
contamination in the product, addition of benzyl alcohol ceased when the blue-green color didn’t return, at least to its full strength, after
allowing the reaction mixture to sit for a period of 30 minutes.
 
After being left to sit overnight in an airtight container, the two-layer mixture was placed in a separatory funnel and the lower aqueous layer
removed. The top layer was washed twice with saturated sodium bicarbonate solution, followed by distilled water, and finally partially dried with a
wash of saturated sodium chloride. The pale green upper layer as well as the aqueous layer became immediately red-orange in color after the sodium
bicarbonate solution was added for the first time, though the color of the aqueous layer is noticeably less with each successive wash. The brine wash
came out colorless, though the benzaldehyde layer is still strongly colored.
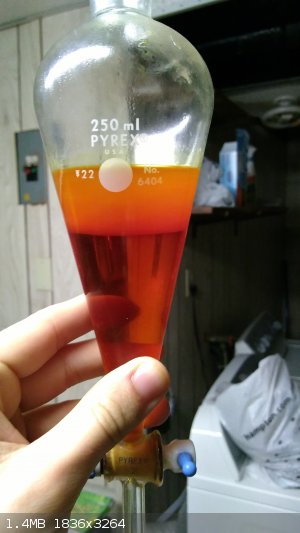 
The crude benzaldehyde, a translucent reddish-orange liquid, was placed into a pre-weighed container with a little anhydrous sodium sulfate and the
weight was recorded as 55.3 grams. If pure benzaldehyde was assumed, this would correspond to an 85% yield! Upon prolonged storage over the sodium
sulfate, the color actually lightened to a golden yellow color, though this isn't pictured.
My plan with this mostly pure benzaldehyde is to purify it using the standard bisulfite adduct formation seen elsewhere. I'm not going to include it
as a part of this write-up due to the fact that I don't have the highest hopes for it; I don't own vacuum filtration equipment. A good example of this
can be found on Chem Player's YouTube channel or elsewhere on this forum.
Analysis
I’m fortunate enough to work in a lab with a GC-MS (gas chromatograph with built-in mass spectrometer) to use at my disposal, so the benzaldehyde
came to work with me the following day. The resulting chromatogram obtained (I’ll be attaching it when I get to work on Monday) contained 3 major
peaks. The largest peak, benzaldehyde, was measured at 92% of the peak area, with an additional peak for residual benzyl alcohol making up another 3%.
The slightly more worrying peak could not be confidently identified from the machine’s database, but the compound suggested it to be benzyloxyurea
(MW: 166.18). This peak didn’t show up in earlier small-scale tests, so I’m not sure if the impurity was introduced as a side product or if I
accidentally introduced it through sloppy lab practices (certainly not out of the question in my case).
Discussion
First I'd like to say that I came upon this method entirely by accident a while back, originally looking to make 3-nitrobenzaldehyde. Hence I didn't
reference a paper for the reaction, nor do I know the mechanisms involved. I can update the thread as per your suggestions, but in the meantime it
serves simply as a guide for preparing benzaldehyde via this method.
Assuming the GC-MS is at all accurate (and it generally has been in the right ballpark where my work is concerned), the yield of this synthesis in
pure benzaldehyde came out to about 79%, which I'm more than happy with. The "benzyloxyurea" peak is troubling to me, as I didn't encounter this peak
in earlier test runs despite both of them being rushed and encountering thermal runaways during the preps. If anyone has any idea what this compound
could be, or what the blue-green intermediate is during the synthesis, I'm all ears.
Given my limited equipment and space, this synthesis, while proceeding without any major hitches or obstacles, could likely be improved in a few ways.
Magnetic stirring and an addition funnel would almost certainly be preferable to hovering over the reaction mixture and swirling it manually, for
example. But the fact that I made do with what I had is a testament to the ease and accessibility of this pathway for the less well-equipped amateur.
Since this is more-or-less my first real write-up on the forum, I'd love your feedback about how to improve my posts in the future, or any concerns or
critiques of the synthesis itself.
[Edited on 2-4-2017 by Amos]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Benzyloxyurea? That seems like an odd impurity. Could it be benzoic acid?
ETA: No, you have a mass spec on the GC, so that wouldn't be it.
[Edited on 4-2-2017 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I found this yesterday, in the course of trying to find a different article that I was looking for:
http://www.tandfonline.com/doi/abs/10.1080/00304940509354991...
I'm guessing that this reaction produces water, hence the need for the P2O5 in the reaction I linked to. The nitric acid is obviously the oxidizer.
It seems oxidation of benzylic alcohols is right up there with "reduction of nitroarenes to anilines" as far as organic reactions that aren't
particularly difficult.
Really, you can do this reaction with bleach as well, as long as you maintain good stirring and slow addition. A nonpolar layer to trap the aldehyde
also helps.
The green color is probably the brief appearance of nitrous acid (which is blue) combined with the yellow-orange of the NOx fumes. Nitric acid minus
one oxygen is nitrous acid, so the stoichiometry works out, although nitrous acid is usually pretty reactive with alcohols. Maybe benzyl nitrite
decomposes into benzaldehyde after some time? Or maybe it's just one of those weird exceptions that always seem to affect anything attached to the
benzyl carbon?
[Edited on 2/4/17 by Melgar]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's the right mass for phthalic acid- which gets everywhere due to the common use of its esters as plasticisers.
Does it have a fragment at M/Z =149?
https://www.agilent.com/cs/library/applications/si-02100_pht...
[Edited on 4-2-17 by unionised]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Here is other article with some good background, references, and suggested mechanisms. They also show benzylnitrite as an intermediate, but that mass
is off from your impurity, benzyl nitrite would be mw 137 and benzyl nitrate would be mw 153.
The experiment looks pretty good, much simpler than using PDC or another metal based oxidant. And the yield is fine for most work. Good job on the
write up.
https://www.scribd.com/doc/78113590/Benzyl-Alcohol-to-Benzal...
[Edited on 4-2-2017 by Dr.Bob]
[Edited on 4-2-2017 by Dr.Bob]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Phthalic acid gets dehydrated to the anhydride on the GC with this method. It's most likely a compound that is too obscure to be in the library in the
first place, as the MS identifies most common molecules like phthalic anhydride or benzaldehyde with greater than 60% confidence. This was 13% if I
recall correctly.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Is nitrobenzoic acid plausible?
http://file.scirp.org/Html/7-1020108/8fa13627-51c3-40cf-a2c5...
I must admit the loss of hydride looks a bit fishy to me.
Wiki tells me that "3-Nitrobenzoic acid (m.p. 142 °C) is a precursor to 3-aminobenzoic acid, which in turn is used to prepare some dyes. It can be
prepared by nitration of benzoic acid. It also can be prepared by treating benzaldehyde under nitration conditions, a process that initially converts
the aldehyde to the acid."
If there's a mixture of two or more materials that co-elute on the GC the MS will give funny results.
Is the mass spectrum the same from the front of the peak to the tail? (And I'd expect some tailing with an acid like this).
I don't suppose you kept the bicarbonate washes? They might have been worth working up for benzoic acid(s)
[Edited on 4-2-17 by unionised]
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Amos,
Some of your observations are similar to my own while studying the nitration of toluene with cupric nitrate in acetic anhydride or sodium nitrate with
sulfuric acid. These reactions also gave an intensely yellow colored extract with sodium bicarbonate. The color was discharged by the addition of
acid. The thread can be seen at:
http://www.sciencemadness.org/talk/viewthread.php?tid=63393#...
AvB
|
|
|
Chlorine
Hazard to Self
 
Posts: 56
Registered: 26-11-2016
Location: Maine, USA
Member Is Offline
Mood: Brominated
|
|
I'm almost sure it's nitrobenzoic acid+ a minute amount of benzoic acid.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You have a reference for that?
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
That paper is an odd and curious synthesis It uses 65% nitric acid and phosphorous pentoxide (PP) supported on silica gel. Now 65% nitric acid is
35% water and the given stoichiometry by my calculation of 18mmol of H2O per 0.9mmol of PP all PP will be converted to phosphoric acid.
Ignoring the reaction of the PP with the silica gel and the alcohol, presumably phosphoric acid could be used in place of the PP.
What am I missing? The PP is supposed to be a catalyst
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Well done and great write-up.
It would be good to follow-up if possible and let us know how much benzaldehyde you recovered after going through the bisulfite purification process
(distillation without a good vacuum is not really feasible and you end up with a huge amount of oxidation)?
Another method we read about was the use of copper (II) nitrate solution to do the oxidation. It definitely works but we found it always over-oxidises
to too much benzoic acid, or in the case of diluting the mixture, then suddenly causes the reaction to stop and fail. Your method could be a really
convenient and easy one if the yield checks out...
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Since you have access, would it be possible for you to run GC/MS on the initial benzyl alcohol? I have some USP stuff and also some of the KYAntec
stuff. The USP grade is water clear but as you know the KYA stuff is an amber color. I'm wondering what the impurities are in that, and if that
contributed to the byproducts in the product?
EDIT:
If anyone is interested in the potential of this method, check out this paper:
Looks like you can pretty much mix 1:0.625 BnOH:HNO3 of 10% concentration, heat it for a couple hours with a pinch of NaNO2, and get like 95% BzH
yield. Nice!
Attachment: scribd-download.com_benzyl-alcohol-to-benzaldehyde-oxidation-w-nitric-acid-92-yield-certified-diy.pdf (176kB)
This file has been downloaded 1287 times
The paper shows that high concentrations of nitric acid reduce yield. At low concentrations of nitric acid the reaction does not require cooling, and
in fact needs additional heat.
A procedure from the paper:
0.3 mol benzyl alcohol is charged into a flask with 1g NaNO2 and 118mL of 10% aqueous nitric acid preheated to 90°C. This is stirred as rapidly as
possible and kept at 90°C for 4 hours. The final product shows >86% conversion of the benzyl alcohol to benzaldehyde and benzyl nitrite, with a
yield of >95% for benzaldehyde and <1% for the nitrite, with <5% unconverted alcohol.
[Edited on 5-2-2017 by Praxichys]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Praxichys  | Since you have access, would it be possible for you to run GC/MS on the initial benzyl alcohol? I have some USP stuff and also some of the KYAntec
stuff. The USP grade is water clear but as you know the KYA stuff is an amber color. I'm wondering what the impurities are in that, and if that
contributed to the byproducts in the product?
EDIT:
If anyone is interested in the potential of this method, check out this paper:
Looks like you can pretty much mix 1:0.625 BnOH:HNO3 of 10% concentration, heat it for a couple hours with a pinch of NaNO2, and get like 95% BzH
yield. Nice!
The paper shows that high concentrations of nitric acid reduce yield. At low concentrations of nitric acid the reaction does not require cooling, and
in fact needs additional heat.
A procedure from the paper:
0.3 mol benzyl alcohol is charged into a flask with 1g NaNO2 and 118mL of 10% aqueous nitric acid preheated to 90°C. This is stirred as rapidly as
possible and kept at 90°C for 4 hours. The final product shows >86% conversion of the benzyl alcohol to benzaldehyde and benzyl nitrite, with a
yield of >95% for benzaldehyde and <1% for the nitrite, with <5% unconverted alcohol.
[Edited on 5-2-2017 by Praxichys] |
I've actually already run it but haven't taken it off the machine onto my computer yet. IIRC there were small amounts of both benzaldehyde and benzyl
alcohol in the starting material, but any impurities amounted to less than 5%. Probably what you'd expect after storing any benzyl alcohol for a few
years.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Nope, this is the same retention time as benzaldehyde I've made via other methods, the product isn't a solid at room temperature (this would probably
be your first clue...?), and essentially the entire mass of the product converted to a cream-colored solid after treatment with sodium bisulfite
solution. Not to mention I still had a product at the end of the base washes. You might want to read the write-up.
This stuff is benzaldehyde.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by wg48  | That paper is an odd and curious synthesis It uses 65% nitric acid and phosphorous pentoxide (PP) supported on silica gel. Now 65% nitric acid is
35% water and the given stoichiometry by my calculation of 18mmol of H2O per 0.9mmol of PP all PP will be converted to phosphoric acid.
Ignoring the reaction of the PP with the silica gel and the alcohol, presumably phosphoric acid could be used in place of the PP.
What am I missing? The PP is supposed to be a catalyst
|
Phosphorus pentoxide on silica gel is a common heterogeneous reagent for solventless reactions, since P2O5 by itself isn't very pleasant to work with.
P2O5 coats the silica gel grains, and also traps nitric acid as it gradually turns into gel-like polyphosphoric acid. I'm pretty sure its only
purpose here is to act as a desiccant and a support.
Also, keep in mind, silica gel is a pretty good desiccant as well.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
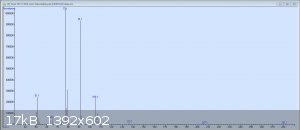
So here's the original GC-MS readout for my crude benzaldehyde. The peaks are in order: Benzaldehyde, the unknown compound, benzyl alcohol, and 3
other tiny peaks each less than 1%: p-nitrobenzyl alcohol, benzoic acid, and 3-nitrobenzaldehyde. It would seem that I was not as considerate in this
preparation as I had been in my earlier test runs. The mass spectrum pictured is from the second peak, which has still yet to be identified.
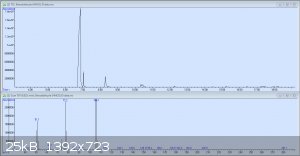
Here's what the mass spec has to say about the second peak. As stated earlier, the compound with the highest probability out of those in the directory
is benzyloxyurea, but it would seem more likely that it is a compound the software doesn't recognize. The other candidates given actually have a
variety of different molecular weights, making it even harder to discern what it might be.

Finally, I wanted to attach this readout from my preliminary test of the oxidation with nitric acid. This was on the scale of 2 grams benzyl alcohol
and 5mL or so of nitric acid; a good deal more acid than I used later on. Over 99% is benzaldehyde with the balance of the peak area as either
p-nitrobenzyl alcohol or noise.

I'm making another go at this synthesis for consistency's sake tonight or soon thereafter on a 1/5 scale of the one in the writeup; I'll post the
results as soon as I've got them.
[Edited on 2-6-2017 by Amos]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It could be a nitrobenzaldoxime. It has M=166.1, it would not be washed away by bicarbonate washes, but I would not expect it to come out of GC so
close to benzaldehyde, and if this is present, then I would expect an even higher presence of benzaldoxime as well (M=121). So this still does not
look as the best candidate.
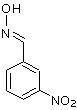
Obviously, the isomeric nitrobenzamide also has M=166.1, but that would surely need way more time to come out of GC. And besides, for it to form,
nitrobenzaldoxime would have to be an intermediate.
If anything isomeric, then I would rather consider a nitroxime as the most likely candidate:
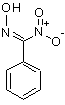
That would explain the absence of plain benzaldoxime. However, I'm not so sure it would not be washed away by bicarbonate washes (I would expect its
pKa is somewhere between 4 and 8). You could try washing a sample with 1M aq. NaOH and run it on GC-MS to check if that peak gets diminished.
Can you post the MS of the second peak? The MS you posted is for the peak at 6.8 min, which is the benzaldehyde peak.
[Edited on 6/2/2017 by Nicodem]
Just found out that nitroximes are called "nitrolic acids".
[Edited on 7/2/2017 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Ack, my bad. Here's the MS of the unknown peak; I don't know how useful it will be.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Beautiful and inspiring work Amos !
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That looks like benzyl alcohol over the tail of benzaldehyde's peak. It is not the mass spectra of the unidentified M=166 impurity you talked about.
Are you sure you didn't mix up the order for the second and the third peak, and the unknown impurity is actually the third peak?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Nicodem  |
That looks like benzyl alcohol over the tail of benzaldehyde's peak. It is not the mass spectra of the unidentified M=166 impurity you talked about.
Are you sure you didn't mix up the order for the second and the third peak, and the unknown impurity is actually the third peak?
|
I'm 100% positive the 3rd peak is benzyl alcohol; I've seen it show up at this retention time in several samples before. There's nothing conclusive
that says the molar mass of peak 2 is 166; that's simply the mass of benzyloxyurea, which was the "best match" the MS's directory contains. As you can
tell from this problematic-looking mass spectrum and from the list of suggested compounds (which have wildly varying masses) on the readout I posted
earlier, the MS really has no idea what molar mass the compound has. Hence why I would like to re-do the experiment and verify that the synthesis can
be done without producing this extra impurity, as I managed in previous attempts at smaller scales.
[Edited on 2-7-2017 by Amos]
[Edited on 2-7-2017 by Amos]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Amos  | | I'm 100% positive the 3rd peak is benzyl alcohol; I've seen it show up at this retention time in several samples before. There's nothing conclusive
that says the molar mass of peak 2 is 166; that's simply the mass of benzyloxyurea, which was the "best match" the MS's directory contains.
|
I misunderstood. I thought you deducted the mass peak from the spectra (N-benzyloxyurea is no reasonable candidate for an impurity, so I could not
understand why you brought it up, unless it fitted the mass).
Then the obvious choice for the second peak is benzyl nitrite (M=137.1). The mass peak is there, although very small, but that is to be expected, as
benzyl nitrite should easily fragment to the same fragments as benzyl alcohols (91, 51). It is an intermediate in the synthesis, so its presence is
normal. The retention time is also reasonable for its physical properties (in between benzaldehyde and benzyl alcohol).

…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by Amos  | | I'm 100% positive the 3rd peak is benzyl alcohol; I've seen it show up at this retention time in several samples before. There's nothing conclusive
that says the molar mass of peak 2 is 166; that's simply the mass of benzyloxyurea, which was the "best match" the MS's directory contains.
|
I misunderstood. I thought you deducted the mass peak from the spectra (N-benzyloxyurea is no reasonable candidate for an impurity, so I could not
understand why you brought it up, unless it fitted the mass).
Then the obvious choice for the second peak is benzyl nitrite (M=137.1). The mass peak is there, although very small, but that is to be expected, as
benzyl nitrite should easily fragment to the same fragments as benzyl alcohols (91, 51). It is an intermediate in the synthesis, so its presence is
normal. The retention time is also reasonable for its physical properties (in between benzaldehyde and benzyl alcohol).

|
Great! I appreciate your expertise on the matter; if benzyl nitrite is the impurity, perhaps the benzaldehyde yield can be increased and made more
pure if part of the workup includes an acid hydrolysis step. When I've got more nitric acid perhaps I'll give this another go.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Ogata Y, Sawaki Y, Matsunaga F, Tezuka H. Kinetics of the nitric acid oxidation of benzyl alcohols to benzaldehydes. Tetrahedron, 1966: 22(8),
2655-2664.
"A probable mechanism is discussed, which involves a rapid and reversible coupling of α-hydroxybenzyl radical with nitrogen dioxide followed by the
hydrolysis of α-hydroxybenzyl nitrite"
http://www.sciencedirect.com/science/article/pii/S0040402001...
This was in conditions with 10% HNO3. A high concentration implies that not as much water will be available for the hydrolysis of the nitrite.
Basically... this confirms Nicodem's comment.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
| Pages:
1
2
3 |