ryborg
Harmless

Posts: 3
Registered: 5-1-2017
Member Is Offline
Mood: No Mood
|
|
Etching copper in copper sulfate - orange precipitate
Hello everyone,
I'm a printmaker experimenting with some less toxic means of making prints, specifically galvanic etching user copper sulfate. The procedure I'm
following is largely outlined here:
http://www.nontoxicprint.com/electroetching.htm
I'm using copper sulfate pentahydrate bought from a local farm supply store. You may know it as Root Killer or something along those lines. Its
probably not lab grade copper sulfate, but I figured it would be good enough to get started with.
When I follow the procedure I am able to successfully etch plates, but my electrolyte solution ends up with an orange precipitate. I'm trying to
identify exactly what it is. My guess is Copper(I) Oxide, but I'm not sure. I'm hoping someone can confirm or deny my suspicions. I've used a
galvanized steel mesh and a stainless steel mesh as my cathodes. I thought the iron in the steel might be part of the explanation for the sludge, but
again, I'm not an expert chemist.
I want to dispose of all waste from the procedure in an environmentally responsible way.

The setup.

Some sludge starting to appear after several uses.

Filtering out the electrolyte

The sludge in question. Copper Oxide?
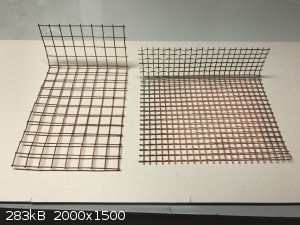
Copper plated onto the different meshes. Galvanized steel on left (basically chicken wire) and stainless on the right ordered off amazon.com

A small test plate etched using this process.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I think you are almost certainly right; it's copper (I) oxide.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
@ ryborg, hey now that's really neat process. what kind of stylus or what-have-you do you use to grave the first one? the reading I did indicated it
is dipped in a resist and that is etched through manually so the process can work on the metal. from then on you can just use that one with etched
depressions to mask the next for doing multiples.
reminds me of a process of inking a whole plexi sheet and subtracting from it manually before rolling it under a paper. forgot the name, only got to
try that a couple times in community college art. it was really fun and way different than previous medias.
I suppose one could silkscreen the resist also. back on topic though, I would definitely agree with unionised. the other metal meshes being
cathodically protected would only tend to deteriorate on standing without charge applied. maybe try a borax bead test for fun? but the precipitate
would have to be well washed of the etching solution of course, already having copper there. probably better ways to confirm
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Neat project. Welcome to ScienceMadness.
For disposal of copper solutions, I generally add baking soda to precipitate green/blue basic copper carbonate. The leftover solution is sodium
sulfate, harmless to pour down the drain. The copper carbonate I keep as a convenient source of copper, but you should be able to throw this away with
regular trash too. When incinerated it will form CuO; essentially copper rust.
You could also try to recover the copper: for reuse in etching solutions, dissolve the carbonate with sulfuric acid to reform your copper sulfate. To
get metallic copper for recycling or other uses, cement out the sulfate solution on a more active metal like zinc or aluminum or add ascorbic acid
(vitamin C) for good quality very fine Cu powder.
Copper chemistry is fun! It's a great entry point to the chemistry hobby.
Great first post by the way - lots of good detail and pictures. Hope you stick around.
[Edited on 1-6-2017 by MrHomeScientist]
|
|
|
ryborg
Harmless

Posts: 3
Registered: 5-1-2017
Member Is Offline
Mood: No Mood
|
|
From what I've read from several safety data sheets, copper oxide still poses a threat to marine life and the recommended disposal method is through a
waste disposal service.
There is a household hazardous waste disposal site in town, but their list of accepted materials isn't very specific. They have a general list of
things like motor oil, detergent, glue, etc, but don't list actual chemical compounds. I tried calling them today, but it turns out they aren't open
on weekends, and I'll have to wait until Monday to actually talk to someone.
Generally speaking, I shouldn't have to frequently dispose of the electrolyte, since the copper plate being etched will continuously re-supply the
solution with copper. Its a nice feature of this particular process - it doesn't lose its bite like a lot of acidic solutions. But if a precipitate
regularly forms, due to impurities or by other means, I may have to deal with that more often.
So, if really am dealing with Copper(I) Oxide, can I safely throw it away in the trash? If my local recycling center will accept it, would the be
preferable?
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
You have all the means, just save it, dissolve with HCl or H2SO4 and plate it out later. No disposal is good no?
Just be sure your filtered solution is neutralized with hydroxide or carbonate before dumping. Personally I'd leave it to evaporate instead of puring
to waste system. And would use sulfuric acid as HCl gas tends to rot metal if it is frequently used/stored in one location.
Maybe cement it out on iron scrap, wash the scrap, neutralize solution then evaporate that.
Id still like to know what you carve the resist with on origonal plates.
Btw,... Woohoo 1000 posts
[Edited on 8-1-2017 by violet sin]
|
|
|
ryborg
Harmless

Posts: 3
Registered: 5-1-2017
Member Is Offline
Mood: No Mood
|
|
There are a couple things I've used as the resist.
1. Hard ground/asphaltum (waxy, tar-like material)
2. Oil based relief ink
3. BIG (a less toxic product designed to make a soft or hard ground)
While I was in university I learned to use hard ground and asphaltum for protecting the plate surface while etching (http://shop.takachpress.com/Graphic-Chemical-Universal-Etchi...). This stuff takes practice to apply thinly with a brush, and it needs to dry for
24 hrs before its ready to draw into. As far as I know, this resist will survive in just about anything. In school we used a hydrochloric acid and
potassium chlorate mixture.
I didn't try the other two resists until later. I started using them when I started searching for less toxic etching methods I could easily use in my
home studio (aka basement).
I tried the oil based relief ink, mixing linseed oil to get the desired consistency, and added cobalt drier to speed drying. I rolled it on with a
roller and then dropped the plate on a hot plate to make things dry faster still. The trouble with this resist is it seems to flake off a little if
you etch for a long time. I usually like long, slow etches so this was a bit of a problem for me. To remove it I would soak it in vinegar for a while,
which was also time consuming.
Finally I tried BIG, which is short for Baldwins Ink Ground I believe (http://shop.takachpress.com/BIG-Etching-Ground-75ML-p/big-gr...). There is a good video demonstration linked on that same page. This stuff works
the best in my opinion. Its hard and ready to use after about 6 minutes on the hot plate, and its easy to remove. It holds up well in copper sulfate
and ferric chloride. I'm not sure how it holds up in hydrochloric acid (if at all). Of the three, its definitely my favorite so far. You can roll it
on really thin, and is ready to use same day after a couple minutes.
Here are some more pictures for fun

Relief ink resist combo

Special made resist product, and the ink I've been using on the right

Some tools of the trade. Most of the drawing I do with the needle on the far right.

The test plate actually printed through the press
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
NICE! Thank you for that. Oddly enough I was an art student in college before switching to chem (and later quitting). Still have a love for art I
guess, just don't draw nearly as often, havent painted in over a year 
This would be combining my love of electrochem and art. Already have a ton of copper sheet for hammering on. Have you tried it on thin copper foil?
Bet it could make a nice backlit art piece. Thanks for giving me some ideas and sharing such a cool process. Have to give it a go when I get some
free time
|
|
|
NitratedKittens
Hazard to Others
  
Posts: 131
Registered: 13-4-2015
Location: In the basket with all the other kittens
Member Is Offline
Mood: Carbonated
|
|
Quote: Originally posted by ryborg  | From what I've read from several safety data sheets, copper oxide still poses a threat to marine life and the recommended disposal method is through a
waste disposal service.
There is a household hazardous waste disposal site in town, but their list of accepted materials isn't very specific. They have a general list of
things like motor oil, detergent, glue, etc, but don't list actual chemical compounds. I tried calling them today, but it turns out they aren't open
on weekends, and I'll have to wait until Monday to actually talk to someone.
Generally speaking, I shouldn't have to frequently dispose of the electrolyte, since the copper plate being etched will continuously re-supply the
solution with copper. Its a nice feature of this particular process - it doesn't lose its bite like a lot of acidic solutions. But if a precipitate
regularly forms, due to impurities or by other means, I may have to deal with that more often.
So, if really am dealing with Copper(I) Oxide, can I safely throw it away in the trash? If my local recycling center will accept it, would the be
preferable?
|
The thing you need to do with copper waste is get a suitable container (I use a milk carton) then when you get round to it acidify the waste with a
mineral acid such as HCl then precipitate out copper metal with the more reactive metal of your choice.
Basket of kittens for you ........BOOM
|
|
|