| Pages:
1
2
3
4
5
6 |
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Fulmen  |
That's understandable, you have more than enough to deal with at the moment. Still, for a plant capable of producing practical amounts of acid a
better method for ammonia is needed. Perhaps someone else could start experimenting with the eU2A, by the time they have a working method I'm sure
you've made headway with the catalyst. |
What is wrong with the standard ammonia salt and sodium hydroxide?
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Nothing, but it will consume more chemicals. Urea is both cheap and readily available, and the CO2 doesn't seem to cause any problems.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I alluded earlier to how I like doing experiments in stages. There's a practical reason for that. I think this project has a good start and a lot of
potential, but it's possible that too many variables are being tackled at once.
Many people here have made ammonia at one point or another. It's a pretty easy thing to do. That's not the important part of your process. What's
important, is your use of alternative, cheaper, catalysts for the oxidation of ammonia to nitric oxide. The ammonia can come from many different
sources, even a pressurized cylinder.
If you can store enough of it ahead of time to allow your system to reach steady-state operational conditions, you can make measurements of the
ammonia/air mix and flow rate, residence time on the catalyst, and make a determination of the product (so many grams per hour, etc.). If the reactor
is always ramping up or down, it's difficult to make these measurements.
The "gas-bag" referred to is just a Zip-Lock bag. The plastic on these is somewhat tough, much more so than a garbage bag or something, but it still
has some elasticity. I connect to one by poking a small hole in the inflated bag, and inserting a slightly larger diameter glass tube, maybe a couple
of inches in length. This glass tube is connected to some flexible tubing, and the glass tube is taped down to the bag, like a nurse tapes an IV to
your arm, to keep it from moving around at the bag seal. Sometimes I'll have trouble with the seal, but usually the seal is a surprisingly good one.
A few days ago I put one of these bags together, and floated it on water with a weight on top. It didn't appear to lose any volume overnight. Of
course, something like this can't provide more than a fraction of a psi, but it's an easy way to store a gas and use it at some adjustable rate.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Quote: Originally posted by WGTR  | | Many people here have made ammonia at one point or another. It's a pretty easy thing to do. That's not the important part of your process.
|
For this prototype I would have to agree, but if one wants to scale things up to a decent production volume this becomes a big issue.
Bringing it up at this time could be a distraction, but at the same time the work shows such promise it's impossible for people to stop thinking of
improvements.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't know about Australia but buying or filling a cylinder of NH3 in the US might well bring the DEA a knockin'.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Ok, here's the details of the latest run.
Catalyst: 1.0gNiO on 10.8g house brick support, screened to pass 7.0mm square mesh and retained on 2.0mm square mesh. Used enough to make a 5cm
reaction zone.
Run time: 5hrs.
Condensate collected: 13ml
Tower collection: 15ml
Titrations:
titrated against 0.5M NaOH
1.00ml of sample in 10.0ml of demineralised water, 0.5ml of red cabbage indicator. (what else!)
Condensate: needed 12.6ml 0.5M NaOH to neutralise. Concentration of sample = 6.3M
Tower: needed 3.23ml 0.5M NaOH to neutralise. Concentration of sample = 1.6M
reference sample of commercial 70% HNO3: needed 33.5ml 0.5M NaOH to neutralise. Concentration of sample = 16.7M
 Start of titration Start of titration
 End point End point
As expected, but not what I hoped; its pretty mild stuff coming from the process. But not too far from industrial results- I think older style plants
would get 30-40% from the oxidising chamber.
The visual observations of the oxidising chamber have been noteworthy. Each reaction has so far behaved differently, subtle differences and not so
subtle.

Note the reaction zone, no pronounced glow like the first run using nickel
https://www.sciencemadness.org/whisper/viewthread.php?tid=71...

And not the dense fumes. Condensation appears more prominent but what does it mean? I will have to do some yield calculations next to see the sort of
efficiencies the catalysts are displaying.
The results are not terrible. This was going to be difficult to get a result and I have come further than I expected with this project. I'm now
intrigued by the possibility of making different catalysts, with promoter and activator additives. Also I want to try palladium just to see the
difference between transition metals and a PGM. But increasing the ammonia to get a decent flow rate, and one that can be measured and controlled,
will solve the issue of catalyst efficiency and conversion values.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Do you have any idea/guess of how much ammonia was produced/consumed? From what I can tell you've collected appr 0.1moles of nitric acid.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
All I can say about the ammonia production is that it is slow. Just by looking at the fumes that enter the chamber it's a wisp of a flow rate. So
really, the conversion might be moderate. More work to do.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Luckily there are many ways to measure gas production, I would probably focus on that next. Sooner or later you will need some efficiency data.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I'm not sure when I can do this, or if I'll have the time at all, but I'd like to help design the metering on the input side of the reaction.
The idea is to use gas bags containing ammonia or air, and seal these bags inside of larger chambers/tubes. A bag would be connected to the outside
world through a fitting in the outer chamber. Gas flow could be controlled by a second fitting in the chamber, that would apply slight air pressure
to the chamber. This would force ammonia or air out of the bag, through the output port. A MEMS flow meter on the input port can measure gas flow,
and this can be integrated with time to give gas volume. As long as the pressures are low in the system (fractions of a psi), I don't think much
error would be added to the results. Except for the flow meters, everything else is a DIY construction project, and fairly cheap. Since the flow
meter is separated from the gas mixture by a diaphragm, it doesn't have to tolerate anything other than air.
I thought of pre-mixing ammonia and air into one bag, as this would require only one flow meter. However, I'm not convinced this is a good idea
(BOOM!). Figuring out the flow rates can give an idea of the residence times of the gas mixture on the catalyst as well as the optimum gas ratio, and
then it will be possible to tailor these parameter for best results.
I appreciate the pictures and your efforts at describing the results, by the way. This is turning out to be a very interesting and informative
project.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I like the bag-in-bag design, starting from a known volume you really don't need a flow meter. As a first approximation you could simply fill bags of
known volume using the same setup&power as your experiment.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's a flowmeter that I occaisionally use for metering argon. You can sometimes pick these rotameters up fairly cheaply on eBay. This one reads in
SCFH (standard cubic feet per hour).

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Here's a brief video (greatly sped up, of course) illustrating the rough concept of a bag-in-a-bottle. The Zip Lock bag acts like a flexible
diaphragm, and isolates the gas from the air supply that's driving it. This allows changing the gas flow rate on the fly. There is less likelihood,
also, of an ammonia release into the room if the bag breaks.
Attachment: gas_bag_in_a_flask.mp4 (4.1MB)
This file has been downloaded 1593 times
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
The bag in a flask is an awesome way to do it really, scaleable with ease. The urea water thermal decomposition is slow; my god it's slow. But as a
feed stock you have to concede it's cheap and easily handled. With some collective problem solving the ammonia generator can be made cheaply and
effectively I'm sure.
So how much ammonia was the generator producing per hour? Not a lot. Today I got the generator running, then bubbled the outlet into a volumetric
flask with about 100ml of DM water. After an hour of running the ammonia was removed and switched off. The 200ml volumetric flask was made up to the
200ml mark and titrated against a known concentration of HCL.
The actual procedure was done by taking a 10.0ml aliquot of ammonia from the 200ml standard solution flask and titrating with 0.455M HCL.
0.455M just happened to be the concentration of a 50.0ml pipette of hardware store HCL added to a 1000ml volumetric flask and titrated with the same
0.5M NaOH solution I used yesterday to find it's actual concentration.
10.0ml of NH3 solution was added to a flask with the same indicator as yesterday (red cabbage) and then titrated with 0.455HCl. until a lightly acidic
point was reached.
This was a source of error with the titration as NH4Cl is slightly acidic at low concentrations so the end point needs a judgement as to the point of
neutralisation. I took it to a mauve rather than amethyst which is the pH7.0 point. A low concentration of NH4Cl hs a pH of around 5.
Note: have edited out the calculations until I get better data, I wasn't happy reporting speculation.
[Edited on 4-1-2017 by Chemetix]
[Edited on 4-1-2017 by Chemetix]
|
|
|
Lefaucheux10
Harmless

Posts: 30
Registered: 28-8-2016
Member Is Offline
Mood: No Mood
|
|
Hi guy,
Very interesting post and i will try to built a similar setup
Here a proposition, why not use ammonium nitrate wich is like urea a pretty cheap fertilizer.
As far as i know it causing troubles to distill off nitric acid from sulfuric acid and ammonium nitrate but ....
for running the Ostwald process you can do
NH4NO3 + NaOH = NH3 + NaNO3 + H2O
and the ammount of NH3 passing threw the catalyst can be controled by the rate of addition of NaOH soln into saturated boiling NH4NO3 soln
after you can boiled the resulting solution to obtain NaNO3 wich can be mix with H2SO4 to make more HNO3
with this you only lost the NaOH from the fist step, some enery to boiled of the soln of NaNO3 and some sulfuric acid
very cheap chemicals ! doubled amounts of HNO3
Another think : Why not absorbate the gaz into some H2O2 for accelerate the oxydation of NOX gases ?
Wait for your comments 
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
There is a problem with getting ammonium nitrate these days. In fact just about any nitrate is hard to come by without paperwork and expense. Many
people on this site have thought about and tried different ways of making nitric acid without a nitrate salt probably for that exact reason. Ammonium
sulfate is readily available to generate ammonia by use of hydroxide. Even urea and sodium hydroxide makes ammonia quite rapidly and effectively. But
they are extra expenses and extra steps.
An ostwald style plant is a continuous process, a switch it on let it run kind of thing, so urea and water can be left to boil and decompose without
having to check on it. A reaction with an ammonium salt has to be run and monitored as a batch process, and once generated you need to then find a way
to add it in a controlled way to the reaction zone. Despite WGTRs ingenious bag in a flask technique, I'd rather avoid it if I can.
And you are right about using H2O2, it does maximise the absorption of NOx. I think that step will be one for getting the last bits of efficiency from
the process, I'm concerned about getting the reaction running reliably and in moderate yield for a start. I have no idea how effective these
alternative catalysts are in the broad sense. I've got a lot of research ahead. But I'm close to being able to state yields and operating conditions
for a given catalyst.
I'll keep posting my progress for those interested in this process.
 This is a very sensitive flowmeter I put together from fish tank needle valves and some small irrigation fittings. The fluid is ammonium sulfate soln.
and some food dye. This is a very sensitive flowmeter I put together from fish tank needle valves and some small irrigation fittings. The fluid is ammonium sulfate soln.
and some food dye.
[Edited on 5-1-2017 by Chemetix]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Good job on the flowmeter. As long as it doesn't change, relative measurements can be recorded from it. The calibration can be done later on.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I think this reference shows some results of using Cu/CuO as a catalyst : http://pubman.mpdl.mpg.de/pubman/item/escidoc:741540/compone...
I point to table 1( row 3 col. 5)
[Edited on 8-1-2017 by ecos]
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
An update on progress
I've learned a thing or two so to keep the flow of information going I'll give an update.
Run#5 Ni2O3 black nickel oxide
I have a flow meter decided to try the highest flow rates that will sustain the reaction.
I replaced the ammonia generator flask from a 250 ml to a 1L 1/2 filled, I cranked up the heat to the ammonia generator and had it refluxing quite
adequately, this should be a maximum of NH3 supplied to the reactor. I ramped up the air flow and tried to see the most flow I could supply. A red
colour and heavy fog persisted. As I brought up the air flow, there was a point where the red colour disappeared. After bringing the ammonia
temperature back down and also the air supply, the reaction failed to start again. The catalyst had changed.

This looks to be NiCO3 and perhaps some NiOH. It fizzed with dilute acid.
Nickel is susceptible to being chemically changed
Run#6 tried a tile glaze that did nothing
Run#7 CoO black cobalt oxide
Started the reactor heater, turned on the NH3 generator turned on the air with manometer reading 40mm. A dark red gas began to appear at the outlet
then as the ammonia generator began to reflux a fog appeared. Ran the system with air at 50mm for a few hours then shut down.
If the reaction was producing dark red gas without fog before reflux then the urea decomposition reaction might be generating too much ammonium
carbamate when refluxing. This might be the source of the fog. Ammonium ions get through the catalyst zone unchanged?
I took some of the condensate and evaporated it in a beaker.

Those long clear needles look familiar. It looks like ammonia is getting past in significant quantities when the urea water reaction is refluxing.

Run#8 CoO same as run 7
Cleared the condensate line and flask, refreshed the tower water.
Kept the air flow on 40mm to begin with and maintained the urea reaction below visible gas evolution, no bubbles. Dark red gas without fog in the
oxidation chamber, the condensate flask and the vertical displacement tube, no visible fumes or red gas leaving the tower.
Left the unit run for 3 hrs.
Switched off the reactor but bubbled the ammonia generator into a 200ml volumetric flask with cold water for 1hr with the same air flow setting
(50mm).
Noticed bubbling in the condensate flask.
This would have to be the reaction :
2NO2 + H20 -> HNO3 + NO
Attachment: NObubbles Run8 CoO.mp4 (1.4MB)
This file has been downloaded 1185 times
Left the reaction to continue overnight.
Next day.
Collected 7ml of condensate and 18ml of tower absorption.
Titrated the samples
condensate 8.1M = 0.056n
tower 7.2M = 0.122n
Ammonia generator collected 0.4M x 0.200L = 0.08n.hr-1
3 hrs @ 0.08n.hr-1 = .24n (NH3)
Theoretical amount of HNO3 from NH3 is n(NH3).024 x 11/6 = 0.439n
Yield (0.056+0.122)/0.439 = 0.4
Approximately 40% from the theoretical amount.
My 80% estimate was ambitious. But urea is cheap, and the rest can be scaled.
I need to reproduce these results a few times before I'll call it properly.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Excellent find Ecos.
It does explain why copper is so erratic as a catalyst at atmospheric pressures, I could find no activity for NO conversion with the so called
"copper" scourer I had at the time.
For a start I have no way of telling if the "copper" was pure.
Higher pressure suppresses the formation of the metal nitride which stops the catalyst working. Other metals which have active oxides but resist
nitride formation would be good candidates to screen for this process.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
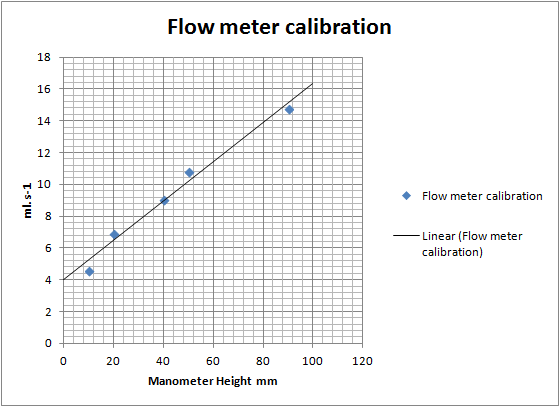
I like your flowmeter. I made a manometer just like it for delta P measurements when I was trying to see if I could use an old HVAC fan from my house
for a fume hood. I also used it when trying to develop a venturi based air-mover. Neither of those experiments produced a suitable air-mover,
however. The delta P vs airflow characteristics were not appropriate.
I'm surprised that your data plot is so linear.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
I'm kind of surprised too. For a start there is no zero, the fish tank valve had no off point, just a slow leak. And I didn't run it a full open
either, that would push the fluid over. The secondary valve controls the scale as I'm sure you know. I set it at something that gave me a small
readable flow and a decent flow at around full scale which was around 90mm height. Values where taken by the time taken to fill a 1L volumetric flask.
The plot could be logarithmic, however, excel fits a fairly convincing linear regression.
If the value for 5mm height turns out to be somewhere around 0.5 - 1.0ml.s-1 I think I'm looking at a logarithmic plot. Which I suspect would be the
case given how these things work.
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I was wondering if some sillica gell dessicant could be used to capture low concentration NO2 effectively, for later release by heat and
production of concentrated acid? make for effective use of H2O2 in a separate container, instead of dumping it in the whole
system and hoping for use vs. decomp. It was mentioned in another thread previously. At the verry least it could be used as a pre-exhaust scrubber,
but I don't recall if there was any issue with NH3 absorbtion.
Ill try to look for that info latr, when more time is available
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
I have a hypothetical that I was curious about for the acid production side of this question.
Could pumping in a close to stoichemetric mixture (for NO2) of ozone and nitrogen increase yield?
It seems a simple water electrolysis system hooked into a decent ozone generator could give you a low pressure source of fairly pure oxygen if
arranged correctly. Pure nitrogen is a harder question, but I figured I'd get the easy stuff out of the way first.
I'd also be interested to know how much more effective H2O2 makes the absorption process, since such an electrolysis device offers the option of
preparing high test peroxide before hand as a feedstock for the acid.
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
If you actually intend on keeping the nitrogen as nitrite/nitrate, lead dioxide is probably the best scrubber you can get easily. However, if you want
to remove all the NOx fumes, I'd recommend sulfamic acid. For convenience, urea can do a decent job too.
I never asked for this.
|
|
|
| Pages:
1
2
3
4
5
6 |