| Pages:
1
2
3 |
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Perhaps you could reduce the effective condenser length in this way:
Run the coolant into the top of the condenser instead of the bottom, and put a valve on the outlet hose for the condenser.
By partially opening or closing the valve you can control how high the column of water in the condenser is(as long as the inlet flow isn't too great).
If this works you could have any given length of your stillhead condenser filled with water by manipulating the valve.
It would be a further complication to add this, but by putting another valve on the condenser inlet, you could not only control the water height in
the condenser, but also control it's rate of flow independently of the height. This would allow you to control the temperature of the coolant in the
condenser and monitor it at the outflow.
Although I suppose it may be simpler to just use the condenser with a conventional bottom to top water flow and to control the input of water with a
valve to control the temperature.
I really don't know if the shorter effective condenser would be better than the warmer condenser. In fact perhaps the warmer condenser would be best
since it would return condensate to the column at a temperature closer to the boiling point of the liquid, which would reduce heat requirements of
the column.
It also might make the column more efficient if it causes less disturbance in the column's temperature gradient.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
I just jumped to answer the original question with my answer here, but I do encourage people to ensure to read the whole thread as I tend to post
things in the simplified concept
Distillation has been one of the more gratifying things I have don from alcohol to other solvents to sulfuric acid.
It is all about surface area, temperature gradient, driving heat.
we can control the temp gradient by controlling coolant flow in a condenser column, or by heat loss from the column when air cooled by increasing the
surface area exposed to free air.
Surface area is fixed, but we can manipulate heat lose with insulation to facilitate getting the temp gradient we desire.
Driving is the heat we input into the system via the boiler, you drive it hard you get a fast distillation but then lots of overlap of your fractions,
drive it gentler you get slower distillation but much better divide of you fractions.
So it comes down to 2 simple variables: Driving heat, column heat gradient.
So the real and only trick to precise fractional distillation is in the configuration of your apparatus.
So a 200mm Vigrux column, a 200mm Allihn condenser then your main condenser.
You set the temp of the Allihn just a bit higher then the fraction you are targeting so only the fraction you want can pass over tot he main one, some
times you just need the one reflux, for higher boiling point substances you can just manipulate the driving heat and let the flask act as the
fractional column.
So you need very little gear to achieve very good distillation quality so long as you under stand there is 2 points that you must control, driving
temp, temp gradient.
For more complex you can add a third variable, Vacuum
And if you want to get serious even more, sweeping gas as the 4th variable, But for the majority of your distillations you'll mostly just have the 2
to deal with and rarely the 3rd
1: Driving heat
2: Temp gradient leading to main condenser
3: Vacuum (This just effects the two variables above)
[Edited on 18-10-2016 by XeonTheMGPony]
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Ditillation theory with reflux
This PDF is part of a write up I did for ethanol distillation. But I explain the design of distillation columns with reflux and the theory behind it.
[Edited on 18-10-2016 by Chemetix]
[Edited on 18-10-2016 by Chemetix]
Attachment: DistillationTheory.pdf (963kB)
This file has been downloaded 604 times
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Bugger
Glass beads never arrived,
I got a full refund and ordered more from a different supplier today.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Balls !
I waited a month for 3mm glass spheres that never arrived, then got a refund,
I ordered from a different Chinese supplier a few days ago,
item marked as shipped, then I get this message:
..........................................................................................
Thanks for your message we just get the response that the item BOLI00036-150G*1;
BOLI00036-150G*2 sent to your counrty have been blocked by our local customs. we are not sure how long will they let the item go. but don't worry, we
promise make every customer satisfied , we know you like our items very much , so we can refund money , , is that OK for you?
Much appreciate for your understanding ,because we can't control the customs.
waiting for your reply .
....................................................................................
How can 3mm glass spheres be of interest to the Chinese export customs department ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Who says the message they gave you is anything closely approximating the truth.
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
If you keep having trouble, Walter Stern Co sells flint and lime glass beads in various sizes on Amazon.
They ship out of New York, so no Chinese customs troubles. But they sell them by the pound for $18.
Don't know if that's a lot more than you're looking for.
I also don't know how long shipping from NY to Oz takes.
I always thought Oz was a part of Washington DC, but most of my literary friends disagree with me on that.
Political symbolism can start a lot of arguments.
EDIT: Oops! YOU'RE not in Oz, that's J_sum1.
New York to England's just a long, cold swim!
[Edited on 26-10-2016 by Maroboduus]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | I waited a month for 3mm glass spheres that never arrived, then got a refund,
I ordered from a different Chinese supplier a few days ago,
item marked as shipped, then I get this message:
..........................................................................................
Thanks for your message we just get the response that the item BOLI00036-150G*1;
BOLI00036-150G*2 sent to your counrty have been blocked by our local customs. we are not sure how long will they let the item go. but don't worry, we
promise make every customer satisfied , we know you like our items very much , so we can refund money , , is that OK for you?
Much appreciate for your understanding ,because we can't control the customs.
waiting for your reply .
....................................................................................
How can 3mm glass spheres be of interest to the Chinese export customs department ?
|
I had a similar experience with glass syringes but they are still advertising them for international sale.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have Balls !
300g of 3mm glass balls arrived - they really were stuck in customs !
300g occupies approximately 180 cc
so I ordered another 100g so that i can fill both columns and have a little spare.
Assuming that they would never arrive, I ordered 2-7 mm 'rose quartz' tumbled chips,
unfortunately the rose colour appears to slowly dissolve in HCl !
and the quartz begins to look a lot like tumbled broken glass
I could have used just tumbled glass chips if there was no pink shit !
I also ordered a new still head adapter to convert to a gas/vapour variable takeoff head

it would be between the top of the fractionating column and the reflux condenser.
I am considering a magnetic stirrer ptfe disc in the side arm, adjusted by an external magnet
.. could be frustrating to adjust
or an adjustable glass rod from above with its end flattened to obstruct the side arm entrance.
... would be frustrating to adjust as the glass rod would have to pass through the reflux condenser.
Whatever method is used, I want it to be chemically inert, vacuum-sealed, and cheap 
Any suggestions ?
P.S. thanks to all who have guided me,
I am unable to stay vertical for extended periods yet, but hopefully next year I will 'publish' some results.
P.P.S. before you ask - I have asked the supplier for a new invoice.
P.P.P.S. it's all been done before - at least I'm learning 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
bitten off more than I can chew (afford) ?
being physically unable to do any testing yet, I've been doodling with numbers for my proposed fractional distillation rig,
and I do not like my results,
(to avoid confusion, my column would be 2x FC7/23 columns stacked vertically (upper and lower) to form one fractionating column)
based on: pot + lower collumn + upper column = 1 + 6 + 6 theoretical plates, with a 19:1 reflux ratio at 1 atm, fractionating EtOH:H2O
starting with anything above 2% EtOH, the top of the lower column (= bottom of upper column) should have >= 90% EtOH at <= 78.4 C
the top of the upper column should be near azeotropic, 95.5% EtOH at 75.1 C.
so
the temperature difference between the top and bottom of the upper column should be c0.3 C
which I believe would require a very precisely controlled upper column heater,
and good heating control or very efficient insulation for the lower column.
so my aspirations have dropped quite a lot ... still learning 
(in this case, calculate first, buy later) 
I'll start with a simpler setup then work my way upwards slowly.
at least now I know why I do not see videos of amateur/hobby fine fractionating columns.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I think it depends on the level of reflux versus the heat loss of the column.
So for example if the heat loss from the column is large compared to cooling due to reflux. Then small changes in the heat loss of the column will
significantly change its temperature and could have a large effect on the reflux level and could stop it completely. No vapour reaches the top.
So the simplest method is to allow the reflux to regulate the column temperature which implies the heat loss from the column must be small compared
heat loss due to the reflux.
Which can be achieved with an insulated column or heated column but not temperature controlled. In effect the column heater only reduces the heat loss
of the column while the boiling point and reflux determine its precise temperature.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
yes, I think that I mostly agree with you,
but if a column heating jacket is at say 5C below top of column temperature
(as recommended by Vogel, page 101, Practical Organic Chemitry, in SM library)
then draughts will change the heating jacket temperature and upset equilibrium,
so many things need to be controlled and monitored (for unattended operation) that I expect this project to be spread over a few years 
meanwhile I'l just slowly develop as I go along ... no more grand projects that I abandon before finishing.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I agree ideally the heated jacket temperature (not the internal column temperature) can be controlled to reduce the effects of fluctuation in ambient
conditions.
The key point is the jacket does not need to be regulated to a fraction of a degree provided that it’s the boiling point and reflux that primarily
control the internal column temperature.
Perhaps the best option would be an insulated column with an outer temperature controlled jacket with a view slot.. This would reduce the need for
good insulation and reduce the effects of ambient conditions while allowing the column to control its temperature.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
O.K. I get you, stable, not neccesarily accurate.
I realise also that my vacuum system (cobbled together pumps, pipes, gauges etc.) and pot heating also need upgrading,
Even physical support structures have to be made, the rig would be my almost height !
I do not need a fume cupboard, I need a fume wardrobe 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
sorry to double post but the surprise arrival of a reflux condenser this Christmas eve has morraly obliged me to push ahead with this project
... thanks  <<< literally and figuratively <<< <<< literally and figuratively <<<
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
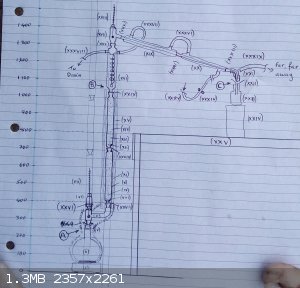
Set up for fractional distillation of c1.65 litres of 56 %ABV EtOH

Spent about two hours setting up
another two hours balancing heat vs. column flooding
my alfresco lab is so cold that even with cooling water at 1 drop per second, I can't get a drop of distillate,
total reflux on the outer wall of the reflux condenser ... to be continued ......
[Edited on 20-2-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
What a set up! Ahh the giddy joy of a nice bit of glassware.
The reflux condenser, which you've already identified as the problem, is too big. It will need heavy insulation in cool weather. And the flow through
it may I suggest, needs to be restricted.
A tee-piece on the inlet of the downward condensers, branching to feed the reflux condenser with an inline valve will be what you need. It keeps the
condensate in contact with optimum cooling but allows the reflux rate to be controlled.
If you weren't wanting fractionation and wanted to just bring over the distillate as quick as possible I'd say the two liebigs in series would be
optimal. But with slow reflux, only one would be needed. For ethanol that is, ether or petroleum spirit would need them both under any condition.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I agree about the two downward condensers ... overkill, but why not ?
I wrapped the reflux condenser in foil after the above post and got a couple of drops in the product condenser.
The main problem has been column flooding,
not achieving reflux rates that I'd expected,
as I was pondering how there could be more refluxing at the top of the column than at the bottom,
the contents of the columns bubbled up into the reflux condenser - the first of several floodings 
I think that the bottom of each column, where the glass spiral is, is initiating the problem,
I will be investigating this ...
This setup is temporary until I get my diy 500ml heating/stirring mantle done.
my secret santa reflux condenser is so much more efficient than the Leibig/West types that I am used to,
all good learning .........
I'll try a simpler fractionation using one un-insulated column and no reflux condenser in the next few days,
just to learn more about the operation of columns,
I'll come back to this setup later .......
P.S. Siphoning cooling water from my upstairs toilet cistern works really well to provide constant cooling water pressure, hence flow rate.
[Edited on 21-2-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Partial takeoff head...that the same piece of glasswar as is referred to as a 'cow' ?
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
No,
a partial take off head is used at the top of a fractionating column
to allow/take a small quantity of product and return most of the refluxed liquid to the column.
It is used to set the reflux ratio.
Industrial systems have a reservoir of condensed vapours from the top of the column, and a little liquid is periodically removed as product.
Amateur systems tend to condense most of the vapours as reflux, allowing a little vapour to pass through the reflux condenser to the product
condenser.
This is the scheme that I am attempting - so far poorly.
a pig (or cow) allows one of multiple receivers to receive distillate,
the cow (or pig) can be rotated to select the next receiver for the next fraction,
only required if the still has to exclude air or completely contain vapours and gasses. (e.g. vacuum distillation, distilation of toxins etc.)
____________________________________________________
I think that 3mm is too small for glass balls for fractionating columns = terribly easy to flood... to be continued .......
P.S. when leaving a fractional distillation to continue unattended outdoors,
check that rain is not due ......

the collection bottle is full and the funnel is filling up,
I blame the rain for the poor photograph.
[Edited on 22-2-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
just an update on my distillation rig
To allow semi-unattended operation I bought six used peristaltic pumps via two eBay auctions from the same seller,
 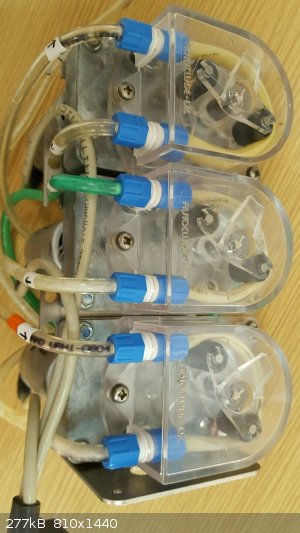
All six came from a cow-milking assembly,
the motors, gearboxes, rollers and housings seem OK
I ordered 1m of 3.2mm i.d. x 1.6mm wall silicone tubing to replace the worn and swolen existing pumping-tubing
and 3x 8A (overkill) pwm controllers for the 12 Vdc motors (£6.90 total).
With 3.2mm i.d. tubing, 1 ml per revolution is pumped
and the motor and gearbox give 125 rpm at 12 V.
I should be able to use one pump for the reflux condenser, one for the product condensor and one as a vacuum pump for reduced pressure distillation.
I was pleasantly surprised by the vacuum pump potential of peristaltic pumps like this,
not a good flow rate but quite respectable pressure reduction
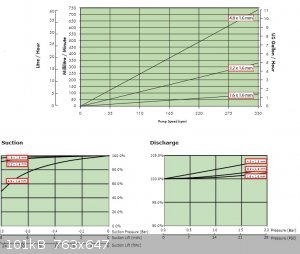
The best part ... all six pumps for £14 inc. p&p 
Now the main thing needed to get up and running is a tall fume-cupboard/wardrobe 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
I am searching for a reflux condenser for a reaction that takes 10-20 hours in alcoholic solution, what kind of reflux condenser will I need? The
volume is 100ml. Reaction temp is at 70C but it may have to go 100C in the end for 30 mins. I'm thinking of buying it from ebay or aliexpress. Thanks.
How do you call the pyrex dishes for the oil bath? I can't find them on ebay.
[Edited on 28-10-2018 by gatosgr]
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
This is the condenser that I got from my clever 2016 secret santa and is shown at the top of my column in the earlier photograph and drawing.
It is efficient but the name is wrong, it is more like a Dimroth than a Graham
https://www.ebay.co.uk/itm/200mm-24-29-Glass-Coiled-Reflux-C...
Slightly cheaper but similar, an actual Graham condenser
https://www.ebay.co.uk/itm/230mm-Graham-Condenser-24-29-Join...
You can use a domestic Pyrex bowl, pot or dish
(some pyrex, especially from USA, is not borosilicate glass, just toughened soda-lime glass)
a glass bowl for an oil bath is nice as you can see through to the flask contents,
but I prefer a metal bowl just because I'm less likely to break it when washing or storing it,
and there is no chance of it breaking during operation.
Any domestic cooking vessel of suitable size and shape will work.
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
As far as fractionating alcohols or other noncorrosive organic solvents goes I must point out that using a glass set up (especially with a
fractionating configuration) is most unpleasant, although visually appealing and will mostly end in shards, tears and sobbing. I highly reccommend to
use a metal setup for these operations. It is sturdy and forgiving....under some circumstances also a lot more economical.
For example if one has access to a lathe and knows how to use it.
One can start out with scrap materials like thusly:
      
And create quite an appealing setup from all of this (the reflux head taking shape):
   
A window section shall be incorporated a the base of the reflux head (in the last picture it has been substituted with a stainless section between the
flanges):
   
The parts dissassembled and a shot of the column packing:
   
It's a work in progress, but it shall be a pilot production/products develpment fractionating column once completed....
The only glass part shall be a 5cm quartz section below the reflux head. The rest is stainless 304. Sturdy and functional without the dangers of
shattering on first contact 
Exact science is a figment of imagination.......
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Although the still that I am building is also a work in progress,
I chose all glass so that I could watch and learn and ponder 
It turns out that ALL parts with upward flowing vapour need to be thermally insulated
so I see nothing without disturbing equilibrium a little by opening thermal insulation to have a peep 
|
|
|
| Pages:
1
2
3 |