| Pages:
1
2
3 |
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
How to adjust reflux ratio ?
I have one of the typical Chinese '500 ml distillation kit'
http://i.ebayimg.com/images/g/w9MAAOSwLN5Wjjaf/s-l1600.jpg
plus an extra Leibig condenser (200 mm eff.) and two fractionating columns (250 mm etff.),
what do I need to adapt my glassware so that I can adjust the take off rate fron the fractionating column - reflux condenser junction?
Cheaper = better 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
The good news is that you don't need anything. You adjust the take off rate by adjusting the heat applied to the boiling flask or the heat lost by the
column. The idea is to set up a thermal gradient.
More heat to boiling flask and thermal insulation around the column = higher take off rate but less separation
Less heat to the boiling flask and more cooling of the column = lower takeoff rate but better separation
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I thought that the idea is to set up full reflux in the column and the overhead reflux condenser,
with minimal temperature gradient in the column, using good insulation or an external heater
then take a small portion to the side condenser for product ?
P.S. I intend to do this to see how well I can do fractional distillation,
how many theoretical plates can I actually achieve vs. theory ?
An exercise in distillation more than general chemistry.
[Edited on 21-9-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
I sometimes add a Claisen adaptor between the column and the stillhead(stillhead goes on the curved side) just to get a little extra condensation
returned to the column. I've even gone so far as to wrap some tubing around the straight upright on the Claisen so I can get extra condensation which
can be closely and quickly regulated with a valve between the tubing and the coolant pump. I know a cold finger condenser could be used there, but my
cold finger is the wrong size for my still setup. Also, I'm not sure how easy it is to regulate the condensation rate with a cold finger.
Prachys, I'm glad to hear somebody say the condensation from an un-lagged column can improve the separation.
I had suspected that, but it seems to go against what it says in most of my lab manuals, which prefer insulating the column.
Perhaps insulation gets more important with really long columns or really slow distillations?
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Oh, I see. You mean like a partial take off head. I read the post too quickly.
The PTOs can be expensive, and I've wondered about rigging one up from standard parts as well.
Here's an idea I haven't tried yet: Put the flask in the mantle at an angle of about 30 degrees off horizontal. Put the column on it with a Claisen on
top and the stillhead on the curved portion of that. put the thermometer adaptor on the straight portion of the Claisen and put the condenser on top
of the stillhead where the thermometer adaptor normally goes. Put an addition funnel on the output of the stillhead where you normally put a
condenser.
Now the condensate when running down the slanted condenser can either enter the collection flask or run back to the fractionating column depending on
how the stillhead is rotated. Since rotating the stillhead so the condensate returns to the column will turn the addition funnel used for collecting
the distillate on it's side you'll need to empty it before rotating the stillhead. (Maybe you could use a large flask instead of the funnel if you're
not collecting too much distillate.)
This won't work with a vacuum unless you can drain the funnel into something like a Schlenk flask that you can evacuate to drain the distillate into
and then break the vacuum on so you can remove it before trying to rotate the stillhead. But of course, using a large flask instead of the funnel, if
possible, would solve that problem.
I hope this is clearer than it seemed to me while I was writing it!
EDIT: writing this out and reflecting on it a little made me realize this glassware setup idea has several real problems with it.
[Edited on 21-9-2016 by Maroboduus]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Maroboduus  |
snip
Prachys, I'm glad to hear somebody say the condensation from an un-lagged column can improve the separation.
I had suspected that, but it seems to go against what it says in most of my lab manuals, which prefer insulating the column.
Perhaps insulation gets more important with really long columns or really slow distillations? |
My understanding is if the column is insulated and the take off is 100% there may be very little condensate flow in column hence very little
fractionation. If the column is then uninsulated and the heat increased to the flask to achieve the same level of take off, there will now be
significant reflux in the column (due to condensation) and hence better fractionation.
Putting it an other way if the take off 100% (no reflux) and the column is perfectly insulated the column is not doing anything.
For the column to work you need a temperature gradient and condensate flow. Those can be provides by walls of the column and or the reflux.
In the Thermos insulated column the reflux provides most of the condensate flow and temperature gradient. Because of the higher condensate flow
through out the whole column the separation is better than the no reflux case. But then how do you arrange the reflux ratio. The tilted adapter seems
the simplest.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
A distillation column can have trays, sieve plates, or packing. For trays it is easy to visualize a series of equilibrium stages but the other types
are equivalent. On each tray equilibrium is approached by some of the heavier component condensing and giving up enthalpy in evaporating some of the
lighter component. Losing heat to the environment through the column wall decreases the efficiency of this process.
In the "Laboratory Text in Organic Chemistry," by Cason & Rapoport, (1950) the authors show how to build columns for difficult separations. These
columns are not only insulated but have external heaters for close temperature control.
IIRC most all the outdoor industrial columns I have seen, such as at oil refineries are insulated.
Perry's "Chemical Engineers' Handbook," 3rd ed, p. 13-53 states "Design of small laboratory columns often presents a number of problems not
encountered in the design of larger units. Enthalpy losses from a small-diameter column can represent appreciable percentages of the enthalpy in the
vapor stream to the column. In such instances vapor and liquid rates are sharply reduced in proceeding up the column. Good practice requires
prevention of such heat losses by the use of electric windings or vacuum jackets."
I normally don't insulate my short (200mm) fractionating column as long as an acceptable separation is achieved. However, I have done a few really
tough separations (bp difference <10°C). In those cases I have used my 600mm column, insulated. I control reflux at the still head with a
condenser/splitter.
[Edited on 21-9-2016 by Magpie]
[Edited on 21-9-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
magpie, thanks,
I thought I understood what is required then got confused.
Following up I found this SM thread
http://www.sciencemadness.org/talk/viewthread.php?tid=10747
sure helps to find stuff when you know what it's called 
The first unit I found cost USD2171.23 ... plus taxes and shipping 
It does seem as if one of the Dean-Stark variations may work ... at my kind of price.
more reading and searching to do ........
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Jekyll
Harmless

Posts: 14
Registered: 3-3-2013
Member Is Offline
Mood: No Mood
|
|
One option to expensive partial-takeoff still heads that I’ve seen recommended is a short condenser, such as a 100 mm liebig, above the column with
a small air or water pump and an adjustable pinch clamp to vary the flow rate and provide provide partial condensation (a water pump also lets you
vary the water temperature in your reservoir). You could try using your 200 mm condenser above one or both of your columns, that would be the
cheapest. A condenser with a drip tip joint would probably make it easier to calculate reflux to takeoff ratios.
Here's a fractional distillation summary and discussion of the technique. I wouldn't agree that Vigreux columns are "virtually useless," but packed
columns do have their virtues.
http://www.q-re-s.com/chem/fractional_distillation.htm
[Edited on 23-9-2016 by Jekyll]
[Edited on 23-9-2016 by Jekyll]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Than you Jekyll! That is the best write-up on fractional distillation that I have seen. What's the source. When I toggle Home I get an error
message.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
I just wish that page said exactly WHAT aspirator that is. Did I miss it?
I get some decent results with my old one, but It'd be great to have some good info on which ones work best.
They're so reasonable price-wise for what they do that It's really worth it to spend a little more for the best.
I know about recirculating icewater for the aspirator, and plan on trying recirculating radiator coolant (maybe with ice or blue ice packs), but a
good aspirator in the first place will mean I don't have to resort to those things as often, and when I do, I'll be getting the most out of them.
Great info though. I often need to see things explained a few different ways before I feel like I've got a handle on them.
EDIT And that link has a section on pyrolysis too! Cool ! (figuratively, of course)
[Edited on 23-9-2016 by Maroboduus]
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Jekyll
Thank You ! ... exactly what I needed.
My intended setup that requires minimum new glassware;
Thermometer
Still Head Adapter - product Leibig condenser - receiver/take off adapter - receiver
Reflux West Condenser
Insulated Fractionating Column(s)
Claisen adapter (just ordered one, £5.75)
Boiling Pot
Very tall in 24/29 !
(why did I buy such short cheap stands ? ...)
Waiting for 3mm glass spheres (not as optimistic about performance now)
and I guess some glass tubing chopping is comming soon .....
[Edited on 23-9-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Jekyll
Harmless

Posts: 14
Registered: 3-3-2013
Member Is Offline
Mood: No Mood
|
|
I'm also getting an error when I look for the /chem/ page, which is odd since it was there when I posted the fractional distillation link.
This organic chemistry primer refers to a "student of organic chemistry." However, I'm not sure if it's from the same source as the fractional
distillation page.
http://www.q-re-s.com/chem/primer.htm
Edit: Here's the link I found earlier:
http://www.q-re-s.com/chem/chem.htm
[Edited on 24-9-2016 by Jekyll]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
More puzzling than his complete dismissal of Vigreux columns is this:
Packing the column with coarse stainless steel 'pot-scrubber' material comes close to Pro-Pak, but the other popular alternatives, glass rings or
short pieces of glass tubing (Raschig rings) will produce compromised results.
Really? Raschig rings are also bad? Also his enthusiasm for the metal "Pro-Pak" defies other guidance here that suggests stainless steel is
useful only for unreactive distillations.
Pro-Pak is just pieces of 316 SS mesh:
http://www.sigmaaldrich.com/catalog/product/aldrich/z210536?...
316 SS mesh is readily available at low cost:
http://www.mcmaster.com/#stainless-steel-mesh/=14asjqr
[Edited on 24-9-2016 by careysub]
About that which we cannot speak, we must remain silent.
-Wittgenstein
Some things can never be spoken
Some things cannot be pronounced
That word does not exist in any language
It will never be uttered by a human mouth
- The Talking Heads
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Wow, I thought it bog standard practice to have a small cold finger at the top of your completely insulated column that has the flow to it regulated
with a hose clamp.
Your reflux ratio can then be tuned from 'full noise' (no flow in the cold finger) to almost complete reflux (full flow through the cold finger)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hmm. I thought it was all fine and froody with just a vigreux column.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Planned Distillation kit Setup ... please criticise
Based on my existing knowledge and glassware, this is my planned distillation rig;
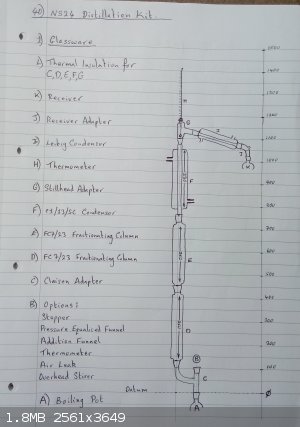
The Claisen adapter and 3mm glass spheres are on order, the rest is existing.
The two condensers have independant flow control
Initially the insulation will be glass wool, I am considering column heating.
I imagine starting with both condensers at high flow
then setting the pot heater to give maximum reflux without choking/flooding
then reducing the flow to the reflux condenser until the thermometer gives a sensible reading and product (very slowly) is collected.
First test will probably be just water
then I want to try to separate Toluene (bp 110.6) and Benzene (bp 80.1)
(I need to get benzene, maybe by distillation from an OTC product ?)
ALL CONSTRUCTIVE CRITICISM WELCOME / WHAT HAVE I OVERLOOKED
EDIT: two relevant documents sent to me by DJF90 (thank you 
Attachment: Packing materials for fractionating columns.pdf (1.4MB)
This file has been downloaded 1535 times
Attachment: Simple calculation of theoretical plates.pdf (686kB)
This file has been downloaded 595 times
[Edited on 28-9-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have made a few preliminary heat calculations for my still above;
to achieve 50 ml/minute at 19:1 reflux ratio:
Methanol, 230W to pot + 13W column loss
Water, 2 kW to pot + 50W column loss
98% H2SO4, 260W to pot + 265W column loss
so I will at least insulate the column,
later I may try heating to compensate for column loss.
Is it practical to get 2 kW into a 500 ml rbf ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | I have made a few preliminary heat calculations for my still above;
to achieve 50 ml/minute at 19:1 reflux ratio:
Methanol, 230W to pot + 13W column loss
Water, 2 kW to pot + 50W column loss
98% H2SO4, 260W to pot + 265W column loss
so I will at least insulate the column,
later I may try heating to compensate for column loss.
Is it practical to get 2 kW into a 500 ml rbf ? |
I have a 3kW electric kettle with a capacity of 1.7l. at full boil the water volume increases by about 50% due to the vigorous bubbling accompanied by
splashing and lots of vapour. I guess that 2kw into say 250ml of water would bubble out of a 500ml flask. A liquid with a lower boiling point and
lower heat of vaporization than water would be more violent. So I think 2kW into 500ml is impractical.
An other way to look at it is the volume of the vapour 50mL of liquid turned into vapor has very approximately 1000x the volume. so thats 50l/minute
or approximately 1l/s pushing out of just 250ml of liquid. You may need a 5 or 10l flask or a very very effective splash head. I don't see your column
or condenser handling that volume either.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
OOPS ! ... well spotted,
sorry, I meant to write 50 ml/hour product at 19:1 reflux ratio,
After re-doing my calculations, now based on predicted maximum column capacity of 15.5 ml/minute liquid boiled;
Water: 583 W heat to pot, 182 W column heat loss if un-insulated
Methanol: 215 W to pot and 96 W column loss.
So my column must be well insulated, or heated.
allowing 1.5 hours for setup, warmup and clean up,
at 19:1 reflux ratio the maximum production rate would be 1 litre per day 
I now realise that I can only collect different fractions if no vacuum is involved
and I want to change the glassware at the top of the column to allow the product condenser to be vertical,
making storage and mechanical support easier.
Basically, I am learning how Vogel evolved his still ...... 
a little bit of knowledge can be frustrating ...
I think that I shall just use this proposed distillation setup in lash-up mode to get more experience before attempting a 'nice' build.
I forsee many thermocouples being involved.
... Mk.II sometime in the future .....
P.S. I think that I fit the 'over-optimistic noob' profile quite well 
P.P.S. For column insulation I am considering this http://www.ebay.co.uk/itm/271545073821?_trksid=p2060353.m143...
any reason not to ?
[Edited on 6-10-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Hope this helps, but here is my reflux controller with a cold finger and thermocouple well; in one pic you can see the control taps for selecting how
much goes into the finger or the condenser. On a good day it can do over 50:1 with something like cineole at 12mmHg, the moon ascendant in
sagitarius...wind from the East, that sort of magic that somehow works on the day, 10:1 with something like ethanol on average-no vacuum.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Damn page with its' two types of "post" options...I'll get the hang of it- here are the pics!
 
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Power calculations are based on specific heat of vaporisation of the liquid condensing. For example- Say 50:1 reflux ratio. 50 drops of methanol (say
1ml each) Std enthalpy change of vaporization, ΔvapHo MeOH +38.278 kJ/mol. 50ml is approx 40g, is approx 1.24n. should be 1.24*38.278kJ= 48kJ. At 2
drops a second there should be 48kJ over 25 seconds
48kJ/25s = 1.9 kW. So your heat source and cold finger should handle at least this power requirement for this reflux condition (i've ignored the 1 ml
that comes over, it's tiny by comparison). When designing distillation equipment I favour the McCabe-Thiel method. Practice has taught me that for
small set ups, 19/26 joints for example, theory means very little. You have an idea of what to expect but the reality is you deal with what the set up
gives you in practice.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thanks for the photo' and info.
but
you have given me more questions than answers 
based on your actual setup vs. my untried theoretical setup;
. my Leibig has a lot more surface area than your cold finger, any problem ?
. why do you not insulate your column ?
. is your packing porous, if so why ?
. what is the steel wool at the top of the column for ?
I ordered my glass spheres via eBay 5Sep. and still not arrived, if not here by Thursday I'll start a claim 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Ok, I've used an uninsulated column because it has a sort of default amount of heat loss all by itself, that means the finger doesn't have to do all
the work. Your leibig by itself is a lot of cooling, maybe too much, you might just end up sending everything back down the column even with very
little flow through the jacket. It presents the system with a very high gradient of heat at one section of the set up, I think I prefer a more
constant heat gradient over the reflux media (in the column) and the finger controlling the last bit of vapour about to come over.
With a liebig you have to really power the flask with enough boiling to get through the huge cooling the condensor can give you. It becomes a brute
force game of boiling vs cooling rather than allowing the column to experience the greatest amount of area exposed to evaporation and condensation
thus giving the high plate count. Flooding the reflux column just sends rivers of liquid down the path of least resistance with little chance of
taking that portion of vapour that was on the cusp of condensing back down the column. In this arrangement I've use clay balls used for grow media
that have very high surface area and filled with pores. More like a HPLC particle. The stainless steel wool just keeps it in place and also stops the
drips from the cold finger from finding a single path to flow down the spheres.
There's a sort of art and feel to designing glassware that the formula just can't give you.
|
|
|
| Pages:
1
2
3 |