| Pages:
1
2 |
bodo man
Harmless

Posts: 5
Registered: 25-8-2016
Member Is Offline
Mood: No Mood
|
|
nano ammonium nitrate/aluminium mix.. can it exceeding CL-20 ?!
by calculation ammonium nitrate-36% aluminium can generate almost 2.5 times energy generated by TNT !!
this is the equation :
10 Al + 6 NH4NO3 = 5 Al2O3 + 6 NH3 + 3 N2 + 3 H2O + 11.16 MJ/ Kg
TNT generating between 4.2 - 4.6 MJ/Kg only !
so, if we using very fine nano aluminium and ammonium nitrate powders (<20 nm size) , and putting the mixture in a very tight moisture proof
container can it be a very powerful cheap explosive exceeding CL-20 and equal to a very expensive octanitrocubane ?!
If it can’t work then what about using another nano-sized oxidizer ?!
sorry for my bad english
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I would think the stoichiometry would be:
2 Al + 3 NH4NO3 = Al2O3 + 3 N2 + 6 H2O
Also, there might be storage stability issues with this mixture. Energy density, while important, isn't the only thing that determines the quality of
an explosive.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
You would get even more energy/kg if you balanced wel your equation...NH3 is a fuel and as such contains a lot of energy.
2 Al + 3 NH4NO3 --> Al2O3 + 3 N2 + 6 H2O
On a theorical level if you could do at the atomic level a perfect mix of Al atoms and NH4NO3 molecules...then you would probably get a very powerful
energetic material.
Sadly theorical is far from reality where such mix would be spontaneously inflamable and incredibely sensitive. Such Al dust would even be inflamable
from open air...
Also a kilo of pure C, H2 or even CH4 (or benzine) would produce even more energy...but are not really interesting/practical explosives.
[Edited on 25-8-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Emulsions fulfill this criteria. They are mixed at the molecular level. It is AN dissolved in water with a fuel added (diesel) and then emulsified.
This produces intimate contact at the molecular level. Sometimes Al is added also, but only up to 10 percent, much more can cause unwanted hydrogen
gas ( if blasting, you don't want hydrogen leaking out of the rocks you just blasted.)
These have a VOD of about 6000ms. And their densities don't come close to the 2.0 of CL-20. I fact these mixtures need microballoons for reliable
detonation.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | Emulsions fulfill this criteria. They are mixed at the molecular level. It is AN dissolved in water with a fuel added (diesel) and then emulsified.
This produces intimate contact at the molecular level. Sometimes Al is added also, but only up to 10 percent, much more can cause unwanted hydrogen
gas ( if blasting, you don't want hydrogen leaking out of the rocks you just blasted.)
These have a VOD of about 6000ms. And their densities don't come close to the 2.0 of CL-20. I fact these mixtures need microballoons for reliable
detonation. |
They also contain a lot of water what tempers the heat of explosions and thus the gas expension --> less work
A gelous stuff (gelifier) is incorporated in a way to keep the Al and the microballoons suspended homogeneously...otherwise they would sediment
(concentrate into the lowest part).
VOD is between 3000m/s to 6000m/s but the last contains extra boosters like NM or NG...
[Edited on 25-8-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
if you go for very small particle size of Al (nm) it will be very very active. a drop of water will make it release heat. ALICE propellant is a prove
to that.
AN is very gyroscopic and this make the mix not stable with Al in nm
if you add Al and AN in ball miller and keep it running till you reach nm, you definitely make a very very sensitive mixture. it will not be stable as
well.
watch this video to see how active is nAl : https://www.youtube.com/watch?v=iIq9VvZeWKo
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Definitely the performance of the AN/Al mixture (called AMMONAL) is always inferior to the performance of common military explosives such as TNT and
TATB. The calculated 11.16 MJ/Kg value is obtained on the basis that all the aluminium burn to form Al2O3 which in fact is far
from being realized in a detonation. Unfortunately, the aluminium combustion is a slow process, so that the energy released by the oxidation of the
aluminium becomes available much too late to influence conditions in the detonation reaction zone. This has a dramatic effect on the detonation
performance and a lowering of the detonation velocity/pressure will be obtained. Another problem with heterogeneous explosive mixtures is the long
time required for the oxidizer/fuel particle to react. These reactions are controlled by diffusion (or other transport phenomena) and also by the
temperature which may control the ammonium nitrate decomposition. You may increase the detonation velocity/pressure by decreasing the particle size of
the oxidizer and fuel but the performance will always be inferior to monomolecular high explosives. On the other hand, aluminium containing explosives
have very good blast properties due to the combustion of aluminium particles with surrounding atmosphere (i.e. the combustion of aluminium with air is
greatly enhanced due to turbulence created at the aluminium/air interface). At a determined distance from the charge, A higher blast/impulse is
obtained from a metal containing explosive compared to a monomolecular high explosive.
Dany.
|
|
|
Bert
|
Thread Moved
25-8-2016 at 11:09 |
bodo man
Harmless

Posts: 5
Registered: 25-8-2016
Member Is Offline
Mood: No Mood
|
|
nano aluminium/ nano ammonium perchlorate mix , how much strong ?!
if there is a way to produce nano amm. perchlorate powder , then mixing it with nano aluminium powder in a stichiometric mixture , how much it
will be strong ?!
from my calculation the Al/AP mixture can produce 11.6 MJ/Kg :
10Al + 6 NH4ClO4 → 6 HCl + 3 N2 + 9 H2O + 5 Al2O3
metal powders in the nano-sized range are very active , very strong reducing agents and have high surface area , so very high rate of burning is
expected ! very high burning rate means simply strong explosion , am I right ?!
I think if the powders are < 10 nm size the burning rate will exceeding some strong mono explosives !!
the mixture also producing a good amount of gases ( 470 g/Kg mix.) wich adding some good amount of brisance !
can this mixture really being a very strong explosive exceeding HMX , CL-20 and maybe Octanitrocubane ?!
11.6 MJ/Kg equal to 2.5 times as TNT , so if it worked it will be very powerful explosive !!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Same kind of question as your first...same kind of reply! The answer is the same!
By the way your post has been displaced to Beginning section...no doubt this post will be merged with it.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
bodo man
Harmless

Posts: 5
Registered: 25-8-2016
Member Is Offline
Mood: No Mood
|
|
no... the first post about ammonium nitrate , this one about ammonium perchlorate , there is a difference 
also the equation in this post is more reality than the old one 
[Edited on 25-8-2016 by bodo man]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
When it come to performance, there is nothing special about oxidizers/fuels mixtures for the reasons explained in my previous comment here
http://www.sciencemadness.org/talk/viewthread.php?tid=68488&...
An extensive study on AP/Al mixtures has been made by Price et. al [1]. Experimental detonation velocities for a series of AP/Al (95/5; 90/10 and
80/20 AP/Al) were obtained and the results show that the detonation velocity did not exceeded 5 km/s. The mixtures were based on micron-sized AP and
Al.
Reference:
[1] Price D., Clairmont A. R., Erkman J. O. Explosive Behavior of Aluminized Ammonium Perchlorate, Naval Ordnance Laborarory Report NOLTR 72-15,
1972.
Dany.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
why you talk just about theoretical things !
if you have nm particle size , everything will be very sensitive !
not safe mixtures to handle or use.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|

if you continue in making new threads like this. it will end up with a new topic like :
Tritonal will be the best EM if we use nAl   
[Edited on 26-8-2016 by ecos]
[Edited on 26-8-2016 by ecos]
|
|
|
bodo man
Harmless

Posts: 5
Registered: 25-8-2016
Member Is Offline
Mood: No Mood
|
|
thank you for this study , I will read it , but I think a 5km/s is good for the micron sized powder .
considering that the deflagration index for micron sized Al powder is less than 80 compared to 35 nm powder wich can reach 350 easily , that is a
very big favourable difference .
I think also FAOB containing nano Al powder and it is very powerful and energy dense bomb anyway !
|
|
|
bodo man
Harmless

Posts: 5
Registered: 25-8-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ecos  | 
if you continue in making new threads like this. it will end up with a new topic like :
Tritonal will be the best EM if we use nAl   
[Edited on 26-8-2016 by ecos]
[Edited on 26-8-2016 by ecos] |
nuclear bombs will be best EM if we used nano Al  
|
|
|
Bert
|
Threads Merged
25-8-2016 at 20:32 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Somehow, this all seems very familiar. Could you please just go find a nice boyfriend/girlfriend/appropriate antipsychotic medication regime/adequate
drug abuse treatment facility/some other unspecified, but more appropriate oulet for teh crazies- and leave this forum the fuck alone? You are a BORE.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Interesting read, I was actually somewhat surprised to see no effect at all on detonation velocity comparing alex and regular Al powder in PBX
compositions (table 2 and 3). Nano-Al just sounds too funky for that. 
[Edited on 26-8-2016 by nitro-genes]
Attachment: DETONATION PROPERTIES OF EXPLOSIVES CONTAINING NANOMETRIC ALUMINUM POWDER.pdf (355kB)
This file has been downloaded 723 times
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
@nitro-genes, the attached file is considering sizes between 100-200 nm
if you choose particles between 10-5 nm it will be super active and effective!
the shape of the particle also matters as far as i know.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Hi Ecos,
Didn't know particle sizes of 5-10 nm were also available, do you have any reference on the use of this in explosive compositions?
Comparing with emulsions somewhat earlier, what is the average size again for the droplets? Although these fuel droplets may behave more different
than solid particles of aluminium when hit by the detonation wave, they are about comparibly small IIRC. Maybe this suggests that something inherent
to the aluminium makes that the VoD is actually not affected and (surprisingly) even slightly decreased for nano size aluminium compared to micron
sized. Maybe the aluminium oxide formed in the reaction zone is very detrimental somehow and the most influential here.
[Edited on 26-8-2016 by nitro-genes]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
it seems I made a mistake. I got confused by units.
I was talking about range of 5-10 um not nm 
sorry for this mistake !
the smallest particle size I am aware of is 40 nm : http://www.us-nano.com/inc/sdetail/467
[Edited on 26-8-2016 by ecos]
[Edited on 26-8-2016 by ecos]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
No problem, it is confusing especially considering alumina and aluminium are different things and with decreasing particle sizes, this distinction may
further become less relevant anyway 
Would it somehow be possible to reduce the surface tension of molten metals? I had the idea that spray techniques of producing spherical aluminium
would not be effective due to the high viscosity and surface tension of molten aluminium. It would be much cheaper to produce nano-al if this were
possible, maybe using a (afterwards removable) flux of some kind or alloy composition. Any thoughts on whether this is possible?
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Would hardness of the metal powder used matter? Would there be any difference between a very brittle magnalium alloy powder and aluminium alone? Or
would the timeframe during detonation be too short to have any effect? If you would design the most brittle alloy using magnesium and aluminium and
lower speed of sound too as low as possible, would it behave thermobaric like a powder?
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
nitro-genes,
The hardness of the metal or whether the metal is considered compressible or incompressible are all of little significance for the detonation
performance in condensed high explosives. If the metal is treated as compressible this will result in a slight lowering of the detonation performance
because a fraction of the energy available in the detonation wave will be used to compress the metal. However, in highly metallized compositions the
density of the metal may have a tremendous effect on the local brisance of the explosive. The plate dent test is known to be a reliable method for the
determination of the brisance of an explosive. The dent produced by a detonating explosive on a witness steel/aluminium plate correlate well with
available detonation pressure of C-H-N-O explosives. However, when a high density metal such as lead or tungsten is present, a deeper dent is
observed on the witness plate despite the fact that the addition of the metals lowers the detonation pressure. As explained by Dr. Charles MADER [1]:
If the plate dent depth fails to correlate with the C-J pressure, as for the inert metal (e.g. lead, tungsten, etc..) loaded explosives, the
explosive is exhibiting unique isentropic expansion properties.
Reference:
[1] C. L. Mader, Numerical modeling of explosives and propellants, CRC, Boca Raton (Fla.), 2008.
Dany.
[Edited on 27-8-2016 by Dany]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
In my prior research on N2O, I recall some commentary on the extreme temperature that a N2O infused fuel explosion could produce.
Fortunately, a powerful primer is required for detonation, although an electrostatic discharge apparently is responsible for some N2O/fuel (including
solvents) accidental explosions.
See https://www.google.com/url?sa=t&source=web&rct=j&...
A N2O/fuel/NH4NO3/Al mix may have some interesting properties on detonation.
[Edited on 28-8-2016 by AJKOER]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
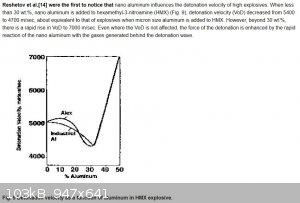
Thanks Dany, is that similar to what is happening in the graph below, very surprising effect that the VoD rapidly goes up with increasing aluminium
content? What causes this effect, the relatively high speed of sound for metals? Interesting phenomena, can't directly fold my head around this. 
Ref --> http://what-when-how.com/nanoscience-and-nanotechnology/meta...
|
|
|
| Pages:
1
2 |