BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Cold Pack Ammonium Nitrate Comparison
I hope I'm not repeating information that is already found in a collective threat on this forum, but I though this may be of use to anyone struggling
to find a viable source of ammonium nitrate, NH4NO3, for lab use.
I wanted do do a short comparison between two brands of instant cold pack derived ammonium nitrate:
-CVS: Locally, I went to CVS, and bough two instant cold packs for 4.79 a piece. These stated "contains ammonium nitrate" on the box.
-Walmart: I then went to Walmart, to find that their instant cold packs contained as stated on the box "Calcium Ammonium Nitrate". Upon a little
Google search I found out that it was ammonium nitrate mixed with calcium carbonate. I did not purchase the wal-mart brand cold-packs since I was only
interested in pure ammonium nitrate.
-Walgreens: Finally I went to the local Walgreens pharmacy, where they had an 8-pack of instant cold packs containing ammonium nitrate for 9.99. This
box did not list ammonium nitrate anywhere on it, but a quick Google search made the decision to purchase easy for me.
For this comparison, two packs of each brand of instant cold pack will be emptied of their ammonium nitrate for an average weight and price
comparison. I will also compare their relative purity by dissolving in water. I plan to acquire more brands of cold-pack to compare in the future.
Here we have the ammonium nitrate from the 2 CVS brans instant cold packs:

Here we see the scale reading approximately 190 grams. My initial impression of the ammonium nitrate is that it's very irregular in prill shape/size,
very off white, leaning towards grey, and has an odd weakly acidic smell to it.
So at ~190 grams with two packs at $4.79/pack, the price for CVS ammonium nitrate works out to $22.89/LB.
Next up is the Walgreens brand cold packs. Here are the contents from 2 of the 8 cold packs in the box.
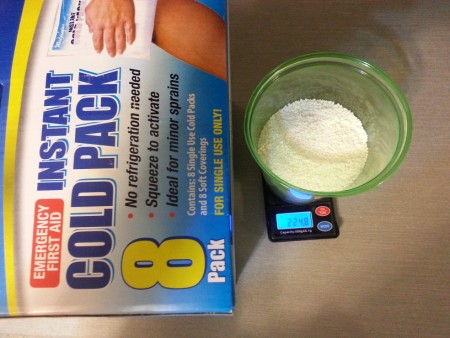
Here we see the scale reading approximately 225 grams. My initial impression of the ammonium nitrate is that it's very regular in prill shape/size,
nearly pure white, and no noticeable odor.
So with 225 grams per two bags and a price of 2.50 per 2 bags (given 9.99 for the box of 8), the cost of ammonium nitrate from Walgreens works out to
approximately $5.07/LB.
To show the visible differences in the two brands here they are on a couple of watch glasses:

And finally, I dissolved three grams of each brand ammonium nitrate into approximately equal volumes of distilled water. The results can be seen below
with CVS brand on the left, and Walgreens brand on the right.

As can be seen above, the Walgreens brand is of a much higher purity, and is far less expensive.
So in conclusion the Walgreens derived ammonium nitrate takes the cake in terms of price at $5.07/LB, and quality, surely suitable for many amateur
lab synthesis.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Your CVS seams to be calcium ammonium nitrate, and your walgreen seams to be urea. You should at least prove somehow the walgreen contain nitrates.
I never asked for this.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thanks for the comparison! The CVS brand definitely looks adulterated with other things, assuming it has any nitrate at all.
The Walgreens brand is what I buy, and I've used it in a bunch of syntheses with no problems at all (including making KNO3 and RFNA). Mine usually
requires filtering to remove some frothy gunk that appears upon dissolusion, but is otherwise perfect for my uses.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
well if it is Ca(NO3)2 then you can do following
H2SO4 + Ca(NO3)2 > HNO3 + CaSO4 (s)
then you can potentially seperate the HNO3 with NC or some other type of filter, squeezing it into a airtight container of glass etc.
interesting that you can even buy an this easily..
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Plante,
The CVS brand states only: "Contains Ammonium Nitrate and Water" although it clearly is not pure ammonium nitrate.
The Walgreens brand has been used successfully in many nitrations and HNO3 synthesis. It is known to be a relatively pure source of NH4NO3.
|
|
|
Trotsky
Hazard to Others
  
Posts: 166
Registered: 6-2-2013
Location: US
Member Is Offline
Mood: No Mood
|
|
Realizing that it's been a long time since this was last posted in, is it possible Walgreens brand switched to urea? I opened two boxes of cold packs
tonight and found, instead of the nice dry, white prills, some off white almost oily feeling prills of much larger size. Upon heating it seems like
ammonium nitrate, but leaves behind a lot of white powder, where ammonium nitrate would break down and leave little residue. I'll have to add some to
conc H2SO4 and see if the usual NOx is released.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
When you heat it, waft a little to your nose. Urea decomposes to solid residues of biuret, cyanates, cyanuric acid, ammeline and ammelides, which
could be the source of your solids but would be apparent by a strong ammonia smell.
Barring that, it could also be calcium salts if they have switched to calcium ammonium nitrate. Pure ammonium nitrate primarily decomposes to oxides
of nitrogen.
|
|
|
greenlight
National Hazard
   
Posts: 754
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
They might have switched to calcium ammonium nitrate which you can still extract the ammonium nitrate from. If they have changed to urea your shit
outta luck.
I had a source of instant ice packs here that contained pure ammonium nitrate and then one day I chucked 6 packs into the trolley and went to cut them
open at home but they had changed the contents to urea seemingly overnight.
Luckily I got a decent amount before the government made the manufacturers change the ingredients.
Be good, otherwise be good at it 
|
|
|