| Pages:
1
..
59
60
61
62
63
..
104 |
Ramium
Hazard to Others
  
Posts: 144
Registered: 3-12-2014
Location: new zealand
Member Is Offline
Mood: Licit fish
|
|
@PHILOU Zrealone and Draconic acid
thanks for the responses. In the end I tried using acetone to force the complex out of solution. When I adding the acetone to the solution containing
the complex, a blue precipitate formed. This substance seems to be insoluble in water but forms a fine suspension which looks like a solution.
I guess this can't be the complex then. Probably just copper hydroxide
insoluble in society
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ramium  | @PHILOU Zrealone and Draconic acid
thanks for the responses. In the end I tried using acetone to force the complex out of solution. When I adding the acetone to the solution containing
the complex, a blue precipitate formed. This substance seems to be insoluble in water but forms a fine suspension which looks like a solution.
I guess this can't be the complex then. Probably just copper hydroxide
|
From memory: the complex is soluble in methanol (my observation). It can be prepared in dry methanol in which sodium and glycerol are soluble.
Presumable the powder is obtain by evaporation of the methanol. I don't know if the sodium hydroxide is required in excess. Probably less so in
methanol than in water.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Ramium  | @PHILOU Zrealone and Draconic acid
thanks for the responses. In the end I tried using acetone to force the complex out of solution. When I adding the acetone to the solution containing
the complex, a blue precipitate formed. This substance seems to be insoluble in water but forms a fine suspension which looks like a solution.
I guess this can't be the complex then. Probably just copper hydroxide
|
Instead of diethylether/ethanol, you may try plain isopropanol.
I don't understand why aceton would drop Cu(OH)2 from the glycerol complex...maybe aceton interacts a lot with glycerol.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Ethyl nitrate
I'm planning to make some ethyl nitrate soon using the method described here
http://www.prepchem.com/synthesis-of-ethyl-nitrate/
The ethyl nitrate is distilled out of the mixture. As a precuation, the nitric acid used is first boiled with urea to remove nitrous oxide.
But to me distilling ethyl nitrate still seems like a very risky thing to do.
Do I have reason to be concerned?
If I treat all nitric acid, with urea as described in the procedure, Will there still be a risk of an explosion?
Thanks in advance
reality is an illusion 
|
|
|
smartgene
Harmless

Posts: 17
Registered: 30-7-2016
Member Is Offline
Mood: No Mood
|
|
Is electroysis the only way to make chlorine
I was wondering is there another way to make chlorine from sodium chloride using a oxidant agent or something that is mild without using hcl or any
acid
[Edited on 1-8-2016 by smartgene]
[Edited on 1-8-2016 by smartgene]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Potassium permanganate will work.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
|
Threads Merged
1-8-2016 at 09:47 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Even without acid as requested?
I know KMnO4 and MnO2 works with HCl or with NaCl and an acid...but the question is about a way to make Cl2 without HCl / an acid.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
smartgene
Harmless

Posts: 17
Registered: 30-7-2016
Member Is Offline
Mood: No Mood
|
|
will peracetic acid work
|
|
|
Jstuyfzand
Hazard to Others
  
Posts: 166
Registered: 16-1-2016
Location: Netherlands
Member Is Offline
Mood: Learning, Sorta.
|
|
Can other metals their oxides be used as a catalyst in the contact process?
(At lower speed/efficiency)
Some sites mention "a metal oxide" catalyst to be used.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I recently obtained a rather large amount of sodium hypophosphite monohydrate. Does anyone know of any interesting reactions I can do with this?
So far, I have:
- it can be used to dehydroxylate alcohols to the corresponding alkanes
- it can be used to reduce diazonium compounds to the unsubstituted derivatives
- with a radical initiator, it can be used to prepare phosphinic acids from alkenes
- it is a useful hydrogen source for transfer hydrogenations
- it has some interesting chemistry with copper salts (forming copper hydride among other things)
These are all very useful and interesting reactions, but my primary reason for purchasing it was as a source of reducing phosphorus which could
substitute for red phosphorus. In addition to uses for hypophosphite itself, is anyone aware of a conversion from hypophosphite salts to phosphorus
or, say, phosphorus halides?
[Edited on 7-8-2016 by Cryolite.]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Lead nitrato hypophosphite complex is a sensitive primary explosive     
US Patent nr 2327867
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Filtering Tinctures - does it remove any active compounds?
I was wondering if tinctures of things like mint, black walnut, raspberry leaves, garlic, etc made with alcohol would have any active ingredients
removed by running through a .45 or 1 micron filter?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by RogueRose  | | I was wondering if tinctures of things like mint, black walnut, raspberry leaves, garlic, etc made with alcohol would have any active ingredients
removed by running through a .45 or 1 micron filter? |
Most usual monomeric organic molecules are into the range of the nanometer...
To give you an idea H2O and N2 are about 1/3 of nanometer.
A nanometer is 10-9 m so about 1/1000 of micrometer what is 10-6 m
So even with a 0.45µm filter you are stil 450 times larger in diameter than a nanometer.
Only very large molecules/3D polymers would be stopped by it except if there is a very big affinity for the matterial of your filter and one of the
consituents of the filtrate.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
shadow
Hazard to Self
 
Posts: 52
Registered: 17-10-2007
Member Is Offline
Mood: No Mood
|
|
velocity of decomposition
I have been reviewing a publication named:
THE DECOMPOSITION OF CITRIC ACID BY SULFURIC ACID
BY EDWIN 0 . WIIG
RECEIVED\ AUGUST 11, 1930 PUBLISHED DECEMBER 18 , 1930
In the article, the author refers to the "velocity of decomposition", which is manipulated by using 4 different strengths of H2SO4.
He holds the temperature and pressure steady, and measures the amount of time that it takes for carbon monoxide to be produced.
I've not seen this measure in any other literature. Is it an outdated or unused measure?
I'm interested in the effects of the different strengths of H2SO4 on yield.
Thanks ahead,
shad
|
|
|
pepe
Harmless

Posts: 35
Registered: 5-6-2016
Location: Memeisland
Member Is Offline
Mood: REEEEEEE
|
|
Perhaps the whole thing is a matter of etymology. Noticing that your cited publication was from the 1930's it very well could have meant velocity in a
slightly different context than the current and precise definition we have arrived at since then. Looking at Websters 1828 dictionary we see that
velocity has two entries. The first being a general relation to rapidity and swiftness and the second which is philosophically (not yet
scientifically) affection of motion by which a body moves over a certain space in a certain time. Both of these definitions make me think that perhaps
velocity was not so much then about the speed of a certain object of study but rather more related to the rate or speed at which things go.
Today the difference between speed (a rate regardless of direction) and velocity (as a vector quantity) is direction awareness. Perhaps this is our
evolved understanding of the term and we suffer from this over complication when reading literature that regarded velocity in a simpler frame.
Much of this is speculation and would be quite cleared up if we could find a dictionary about 100 years newer than my reference but that was the only
version I could readily access.
|
|
|
laserlisa
Hazard to Self
 
Posts: 52
Registered: 5-2-2016
Member Is Offline
Mood: No Mood
|
|
I have dried some ethanol using 3Å molecular sieves, and the ethanol has gotten quite cloudy because of what I assume is sieve dust. Does anyone have
any tips on how to remove the cloudiness?
Also this zeolite dust is relatively inert I assume, but are there any reactions in which this dust might cause troubles?
Thanks
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Leave the ethanol to sit (over the sieves) for a few days. The dust will settle, and the dry ethanol can then be decanted off.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I had a runaway when I attempted to distill methyl nitrate ─ ethyl nitrate is higher boiling, has a lower OB and is markedly less brisant than NGl,
EGDN or methyl nitrate!
All things considered, it doesn't have much bang for bucks!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by hissingnoise  |
I had a runaway when I attempted to distill methyl nitrate ─ ethyl nitrate is higher boiling, has a lower OB and is markedly less brisant than NGl,
EGDN or methyl nitrate!
All things considered, it doesn't have much bang for bucks!
|
Following Rudolf Meyer-Explosives,Ed4- p131:
Ethyl nitrate has a VOD of 5800 m/s and a Lead block test of 420 ccm³/10g for a density of 1.1g/ccm³.
Vapour forms explosives mixtures with air at room temperature (lower explosion limit 3.8% Ethyl Nitrate)
It is stil a sensitive and powerful nitric ester...overheating during distillation is forbidden --> cold distillation or under vaccuum and in
minute quantity.
Impact sensitivity must be between 0.2 and 3 Nm based on related nitric esters.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
artchemix
Harmless

Posts: 2
Registered: 16-8-2016
Member Is Offline
Mood: No Mood
|
|
Copper acetate and iron reaction
I made a copper acetate solution and used an iron wire to stir it. As soon as the wire touches the solution a thin layer of a black precipitate formed
on the wire.
I left the iron wire on the solution for sometime and some bubbles appeared and the blue copper acetate solution slowly became discolored. I think
this was a displacement reaction where the copper precipted out as small particles and iron acetate was formed. This is possible because copper is
lower than iron in the reactivity series.
What amaze me the most was that I did not found any records of this reaction online. I want to know if my assumption is correct.
Thanks
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Any copper(II) salt will react with iron. You are correct.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
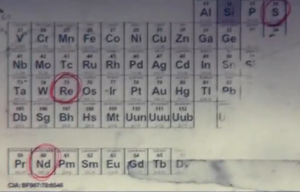
Anyone know how to make the fictional gas, NOVA 6? No, but seriously, could it be a real substance? It is described as follows:
| Quote: |
According to the periodic table, the three basic elements in Nova 6 are Sulfur (S), Rhenium (Re) and Neodymium (Nd).
Nova 6 appears as a dark green gas. Upon inhalation, it causes vomiting, violent coughing, muscle convulsions, bleeding from the eyes, and blackening
of the skin (necrosis), followed by an agonizing death within 10–20 seconds of exposure. In the more refined...form, Nova 6 retains its green color,
albeit with a slight yellow tint, and has a much more instantaneous effect. Once inhaled, the victim suffers violent coughing, nausea, vomiting,
suffocation, bleeding from the eyes, and scaling, burning skin, with death occurring before the body can even hit the ground.[1] This version has a
100% fatality rate,... the gas was tested on infants, causing death within 30 to 40 seconds.
|
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Velzee  | Anyone know how to make the fictional gas, NOVA 6? No, but seriously, could it be a real substance? It is described as follows:
| Quote: |
According to the periodic table, the three basic elements in Nova 6 are Sulfur (S), Rhenium (Re) and Neodymium (Nd). |
|
There is no metal sulphide that could possibly be a gas under ordinary conditions.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Velzee  | Anyone know how to make the fictional gas, NOVA 6? No, but seriously, could it be a real substance? It is described as follows:
| Quote: |
According to the periodic table, the three basic elements in Nova 6 are Sulfur (S), Rhenium (Re) and Neodymium (Nd).
Nova 6 appears as a dark green gas. Upon inhalation, it causes vomiting, violent coughing, muscle convulsions, bleeding from the eyes, and blackening
of the skin (necrosis), followed by an agonizing death within 10–20 seconds of exposure. In the more refined...form, Nova 6 retains its green color,
albeit with a slight yellow tint, and has a much more instantaneous effect. Once inhaled, the victim suffers violent coughing, nausea, vomiting,
suffocation, bleeding from the eyes, and scaling, burning skin, with death occurring before the body can even hit the ground.[1] This version has a
100% fatality rate,... the gas was tested on infants, causing death within 30 to 40 seconds.
|
|
Fictional it is fictional it remains...so unreal yes and as such you could do it fictionally by nuclear fusion...and exposition to gamma-rays from a
super sun just before passing it through a black hole and distilling it out of a whorm hole with a pinch of Jupiter clouds and ice from Europa...
More realistically:
If the solid matter is nanosized, then it could become airbone and remain in suspension thanks to Brownian move of surrounding gas molécules...but on
itself not a gas a such.
[Edited on 18-8-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Hmm..could oxalic acid be used to produce nitric acid, or would it just be oxidized as HNO3 is produced?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
| Pages:
1
..
59
60
61
62
63
..
104 |