IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Tautomers of substituted Pyridines
Hey guys, I'll apologize in advance for the big post!
I'm determined to finish a intermediate organic chemistry course I started a long time ago (it's over but I downloaded all the content) and I've a
doubt about the favored tautomer form of some Heteroaromatics.
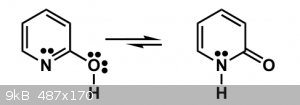
On the webcast, it's said that on the molecule 2-Pyridone we have a tautomerism equilibrium like in the image below, where the pyridone form is
favored instead of the 2-hydroxipyridine form.
Pyridone Tautomerism Equilibrium:
Each tautomer have 2 major contributors for resonance, listed on the image below (for the 2-Hydroxipyridine and 2-Pyridone respectively):

On the 2-Hydroxipyridine resonance forms we have on the left an aromatic uncharged molecule and on the right an aromatic charged molecule. However the
most electronegative heteroatom (Oxygen) is positively charged and the nitrogen negatively charged. This makes this resonance form a weaker
contributor.
On the 2-Pyridone resonance forms we have on the left a non aromatic (8pi e-) uncharged molecule and on the right an aromatic (6pi e-) charged
molecule, where the oxygen is the negatively charged heteroatom, matching the electronegativity character of each heteroatom. This
favors the 2-Pyridone tautomer.
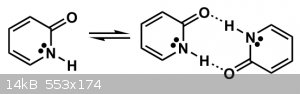
Another factor mentioned was the dimerization of the molecule, which was geometrically favored in the 2-Pyridone form.
So far so good, the doubt comes when the tautomers of 2-Mercaptopyridine are discussed.
Tautomers equilibrium of 2-Mercaptopyridine:
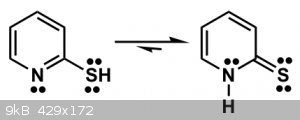
It's known that the thione form (right) is favored. It's not explained as detailed as in the pyridone case why, so I decided to analyse it.
I've drawn the 2 major contributors of resonance on each tautomer. I'll show and discuss them.
First the Thiol tautomer form resonance contributors:
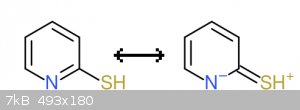
On the left we have an uncharged aromatic (6pi e-) molecule. On the right we have a charged and also aromatic (6pi e-) molecule, with a positive
charge on sulfur and a negative charge on nitrogen.
Now the Thione tautomer form resonance contributors:
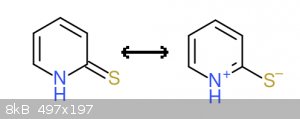
On the left we have a non aromatic (8pi e-) uncharged molecule. On the right we have a charged aromatic (6pi e-) molecule, with a negative charge on
sulfur and a positive charge on nitrogen.
I could make the following assumptions after drawing those:
1) Considering only aromaticity, the thiol tautomer would be more stable than the thione tautomer, since both
major resonance contributors are aromatic on thiol form, but only one contributor is aromatic on thione form.
2) Considering the heteroatoms electronegativity, knowing nitrogen is more electronegative than sulfur, the thiol
tautomer would have the right charges on its charged resonance contributor, being more stable than the thione tautomer.
3) Considering the geometry for dimerization, the thione tautomer seems to have a better geometry for the
hydrogen bonds and would be favored over the thiol tautomer.
Only assumption (3) is in favor of the thione formation, however it's the favored tautomer. Why so?
In the course webcast, the teacher makes it sound like having the negative charge on the sulfur and the positive charge on nitrogen would be the right
charge distribution. Wouldn't the opposite be true considering that sulfur is less electronegative than nitrogen?
Here's a youtube link to the webcast mentioned:
Hydroxypyridine-Pyridone Tautomerism
Thank you in advance!
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
No clues about the reason behind this tautomer preference?
The only reason I could imagine is that the geometry that facilitates dimerization is the key factor, compensating both aromaticity and heteroatoms
electronegativity. But that sounds a bit odd..
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The problem is that nothing in organic is simple. Even if there are five possible resonance structures for tautomers, and four support one form, if
the other one is still better, that will win. It is not just a matter of majority rules. Also, there are often complex rationale for some results,
often involving orbitals and even anti-bonding orbitals which can interact, especially in the case of larger atoms with D orbitals like sulfur.
Also, my thought might be that sulfur, being larger can handle a negative charge better than nitrogen. The key lesson that I have learned is to
look at empirical data. I have had people tell me that science "proves" that bumblebees can't fly, penicillin can't work by the mechanism shown in
books (there is still debate on exactly how it works after 50+ years), thiophene either can or cannot exactly replace benzene as an isostere in drugs,
and many other bits of theory presented as fact.
My key point if that you should examine the actual data and then try to speculate why it happens that way, see if that theory can predict the outcome
of other experiments, provide incite for other experiments, or help you to get to the molecule that you wish to make. If so, great, if not, then
perhaps the theory is incomplete/wrong/misapplied/only part of the answer...
The bottom resonance form would allow me to speculate that the sulfur could react readily with methyl iodide to form the methyl sulfide (methyl
mercaptan). if that is true, that would both help confirm the theory that the right structure exists in a reasonably large amount, as well as help
me design my reactions. But keep in mind that many existing reaction mechanisms, for example, palladium based coupling chemistry, have intermediates
in them that experimentally may only be detected as a small fraction (<1%) of the equilibrium mixture. So while there may only be a trace of one
resonance form around, compared to others, if it is the important reactant, it may still be the only one that matters. Sometimes the trace
configuration/tautomer is a million times more reactive than the majority of the material. So chemistry mechanisms are not always simple or obvious,
and can challenge even experts. I have seen more than a few organic exam questions that are just wrong, in the sense that what they askmay exist in
textbooks, but don't work well in reality. The equilibrium between orthomethyl phenyl Grignards and benzyl Grignards is a good example, I have seen
many questions where one or the other is used, but they often inter-convert, so the real answer is a mixture of products will form.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm not sure about the tautomerism, but the anion of that would be a cool ligand.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The thiol has actually two additional tautomers, with the negative charge placed instead on the 3-or 5- carbon of mercaptopyridine. But the thione has
*three* additional tautomers, with the positive charge on either the 2, 4 or 6 carbon of pyridine.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by clearly_not_atara  | | The thiol has actually two additional tautomers, with the negative charge placed instead on the 3-or 5- carbon of mercaptopyridine. But the thione has
*three* additional tautomers, with the positive charge on either the 2, 4 or 6 carbon of pyridine. |
If you're moving the charge around, that's resonance, not tautomerism.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
|