| Pages:
1
..
35
36
37
38
39
..
81 |
MineMan
International Hazard
    
Posts: 1014
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I am very impressed, I am even wondering if this mixture could be set off by flash powder...it seems very sensitive. From your test I would say KCl03
is a tad more touchy in this mixture.
That had some power... Donier, would you be up to seeing if .2grams of your super flash could set this mixture off?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
ini
I know Dornier super flashpowders. Huge work on deminers the field. In this direction I recommended working on quality NPED detonator on ETN based.
From a practical produce of amount for detonators. ETN is good, maybe best way for initiation of anything. 300 - 500 mg as output segment is certain
for all next attempts. ..LL...
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | I know Dornier super flashpowders. Huge work on deminers the field. In this direction I recommended working on quality NPED detonator on ETN based.
From a practical produce of amount for detonators. ETN is good, maybe best way for initiation of anything. 300 - 500 mg as output segment is certain
for all next attempts. ..LL... |
Isn't MHN more powerful and sensitive than ETN? It should be even better for NPEDs, though mannitol is not so common as erythritol.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
sugars
Well, this is maybe main reason for large using ETN. Mannitol alcoholic sugar is for deminers with source from the hospital. Erythritol is for
everybody in civilisation the world. Thanks huge amount diabetics. Will be impossible for every government restricted sales this sugar. Farmaceutical
lobby is against. It is much a big business. And is every a year a bigger. (huhaha) ...LL...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by OneEyedPyro  | Quote: Originally posted by Laboratory of Liptakov  | I know Dornier super flashpowders. Huge work on deminers the field. In this direction I recommended working on quality NPED detonator on ETN based.
From a practical produce of amount for detonators. ETN is good, maybe best way for initiation of anything. 300 - 500 mg as output segment is certain
for all next attempts. ..LL... |
Isn't MHN more powerful and sensitive than ETN? It should be even better for NPEDs, though mannitol is not so common as erythritol.
|
Following Rudolf-Meyer Explosives book, sensitivity of MN, EGDN, NG, ETN is 0.2 N/m while that of MHN is 0.8 N/m --> MHN is 4 times less
sensitive...
Since 1N = 98gr +/- = 100gr
0.2N/m is equivalent to the fall of:
a 20g weight from 1 meter
a 200 g weight from 10 cm
a 2kg weight from 1 cm
Explosive pressed between two hard metallic surfaces...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NPED
Ha! One Eyed... This is important information. This difference I see firstly. Thanks about it. For system NPED I estimate will be better ETN. However
is necessary say, that impact test is not all for construction quality deflagration- detonation- transfer ( DDT ). ETN is according international
order secondary substance. Not primary. And it is important for next examinations on the field of Non Primary
Explosives Detonator.. ...LL ...LL
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I don't have access to nitromethane so testing the exact same mixture will not be possible. From what I saw in Liptakov's video I'd say it can be
kicked to high order detonation with much less than 300 mg ETN. 200 mg of my best nano flash could very well work. It's not as brisant as for example
lead azide, but does detonate and delivers five times as much energy.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
nano
I tried several times nanopowder according Dornier, but results was weakly, than original. Produce 200mg for me, was so much work, almost madness.
Therefore I recommended ETN as base for NPED...LL..
|
|
|
MineMan
International Hazard
    
Posts: 1014
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Donier, I think your FP would kick it off. Theoretically I think that would be significant because the whole system would contain no primary, or no
pseudo primary such as ETN... I think it would be interesting.
|
|
|
Eosin Y again
Banned troll

Posts: 13
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
What kind of nano flash (KClO4/ferrocerium etc?)
|
|
|
Eosin Y again
Banned troll

Posts: 13
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Some thoughts.
1) Acetic acid + tetranitromethane = trinitroacetic acid?
CH3COOH+C(NO2)4=C(NO2)3COOH+CH3NO2 (which would bubble out of solution.)
2) Ammonium chloride + trinitroacetic acid = dinitroaminoacetic acid?
NH4Cl+C(NO2)3COOH=C(NO2)2COONH3+HCl
These might be rubbish because I am just speculating.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Eosin Y again  | Some thoughts.
1) Acetic acid + tetranitromethane = trinitroacetic acid?
CH3COOH+C(NO2)4=C(NO2)3COOH+CH3NO2 (which would bubble out of solution.)
2) Ammonium chloride + trinitroacetic acid = dinitroaminoacetic acid?
NH4Cl+C(NO2)3COOH=C(NO2)2COONH3+HCl
These might be rubbish because I am just speculating. |
Right! Speculative full of mistakes irrealistic dreams!
[Edited on 15-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
nano
Research nano powders I appreciate very much. But so far, unfortunately, I have not seen one functional detonator based on nano powder. Against this,
NPED on ETN based worked. Hundred exemplars was tested and really it worked. With fuse, with different ratios and different additives. MineMan trying
ETN + Mg + KClO4 and DDT worked this. Results I not see, but belive that worked. If nanopowder hacked 2mm steel plate (hole through) , after it, I
will belive. It will be a small hole in plate, but big jump for all deminers... ...LL ...LL
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Some nano flash powders show incredibly fast DDT and fairly good brisance usually more often associated with molecular primaries, I think some would
be good candidates for use in detonators in terms of raw performance.
What I don't understand is why anyone would want to use a nano flash powder in place of ETN considering that the static spark sensitivity is typically
much worse with FPs, not to mention how metallic fuel/oxidizer mixtures are often not storage stable over time and how insanely hard nano flash is to
make in practical quantities.
ETN is safe yet just sensitive enough to be used in NPEDs, it has shown to be reliable and effective etc.
Why fix what isn't broken?
|
|
|
MineMan
International Hazard
    
Posts: 1014
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
OneEyed, I agree, good points.
Yes LL, that mixture does work, quite well... I might say I am quite impressed with myself!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
simply
Albert Einstein once said: Things should be done, as simply as possible. But not simpler. And I say: things should be done so simply, to fulfill its
purpose... ...LL ...LL
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
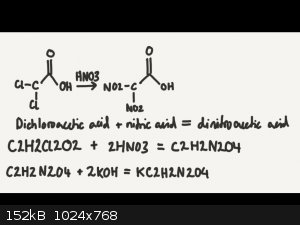
True or false?
And by the same token trichloroacetic acid + nitric acid = trinitroacetic acid?
The above would be better because it would give a higher oxygen percentage, allowing for more explosive, more dense transition metal salts.
[Edited on 16-5-2016 by glymes]
|
|
|
Cryolite
Harmless

Posts: 15
Registered: 20-4-2016
Member Is Offline
Mood: No Mood
|
|
That isn't going to work.
2HNO3 + CHCl2(COOH) -> CH(NO2)2(COOH) + 2HOCl (!)
The (hypothetically formed) hypochlorous acid would then oxidize the substituted acetic acid even further, forming a large mess.
Regardless, Cl+ is a better electrophile than NO2+, and so any proposed electrophilic substitution would not work.
Nitrite, on the other hand, may work, as is the case in the lab-scale preparation of nitromethane from chloroacetic acid and sodium nitrite -- the
nitrite isomerizes to a nitro due to the electron withdrawing properties of the carboxylic acid substituent.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Oh. So 2NaNO2+2HCl+CH(Cl)2(COOH)=CH(NO2)2(COOH)+2HCl?
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
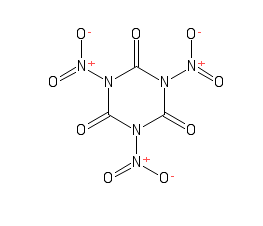
Next please...
C3N3Cl3H3+3HNO3=C3N6O9+3HCl+3H
I can't find any literature references.
[Edited on 17-5-2016 by glymes]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
C3N3Cl3H3???
What is this? This cannot be an existing cyclic compound. We have C3N3Cl3 (cyanuric chloride) and C3N3Cl3O3 (TCCA) but not C3N3Cl3H3.
|
|
|
glymes
Hazard to Self
 
Posts: 53
Registered: 16-5-2016
Member Is Offline
Mood: Fiddly
|
|
Sorry about the typo. C3N3Cl3O3. I was wondering why my reaction didn't make sense!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | Albert Einstein once said: Things should be done, as simply as possible. But not simpler. And I say: things should be done so simply, to fulfill its
purpose... ...LL ...LL |
The K.I.S.S. pragmatism...
Keep It Short and Simple   
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by glymes  | True or false?
And by the same token trichloroacetic acid + nitric acid = trinitroacetic acid?
The above would be better because it would give a higher oxygen percentage, allowing for more explosive, more dense transition metal salts.
[Edited on 16-5-2016 by glymes] |
Oh NO!
You again!
I recognised your DNA!
-Octonitrocubane
-A nitrogen rich explosive
-Eosin Y
-Eosin Y again
You keep on doing the same mistakes again and again!
Write équations correctly please avoid = symbol and gross molecular formulas!
1°)Valences carbon is tetravalent --> you miss a H atom
2°)If your compound do existed it would be a diprotic acid and with a base, the protons would be lost and replaced by the metal...so again the same
kind of mistakes as you usally did under your previous ID's.
3°)Nitration will happen on the acidic H and form eventually dichloronitroacetic acid, such compound will more than certainly decarboxylate into
dichloronitromethane.
4°)The later will maybe disproportionate into chloropicrin and chloronitromethane or nitromethane; or nitrate further forming dichlorodinitromethane
but with a high risk of formation of NxOy and phosgen (Cl2C=O) because geminal NO2 are unstable and easily turns into nitrite esters.
[Edited on 17-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
And magically TADAAAHHHH...
the sodium disappeared from the equation...
Glymes aka the multiple ID poster is also an alchemist magician transmuting sodium to anti-matter...
How is nitromethane done from chloroacetic acid and NaNO2?
Search for the equation and transpose to mono-substitution and disubstitution...
What could be the side products since substitution is not 100% effective? 60%-30% depending on conditions...
The other product is hydrolysable into what?
[Edited on 17-5-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
35
36
37
38
39
..
81 |