| Pages:
1
..
3
4
5
6
7
..
16 |
carrant
Harmless

Posts: 39
Registered: 19-3-2015
Location: Dallas, Tx
Member Is Offline
Mood: No Mood
|
|
These items were included in an auction lot that I picked up.
The auction lot consisted of vortex mixers and these items, which were labelled as accessories.
The only label is on the bottom of the large piece with the two cylinders (second picture).
I visited Ann Arbor Plastics' web page but didn't find any links to their scientific products department.
Ann Arbor Plastics
I've tried to assemble the pieces but they don't seem to fit, so I've been wondering if perhaps they are a collection of disjointed pieces that were
tossed in the auction lot OR maybe there are pieces missing from the set.
   
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
They are parts for liquid chromatography, beloved of biochemists of old.
The red bases attach on either side to a glass tube, have a plastic "bed support" and the glass tube is packed with a gel - usually a dextran that is
selective for molecular size (eg Sephadex, Sephacryl). There should be a thin pastic tube that comes out of the long white "pipes" that go into the
red bits. There is probably the brand name Pharmacia written on some of that stuff.
The plastic base with the two disks recessed in it is a gradient maker. You fill up the highest on one side, and the lowest concentration on the side
with the hole drilled under it. You pop a magnetic stirrer on the lowest side, then draw fluid out. This gives a low-> high gradient that is useful
to elute (salt off) proteins from columns, or to make a variable pore-sized acrylamide gel for electrophooresis.
Nice, reminds me of my youth. Great for protein purification/separation, not much else.
[Edited on 3-4-2016 by Harristotle]
|
|
|
carrant
Harmless

Posts: 39
Registered: 19-3-2015
Location: Dallas, Tx
Member Is Offline
Mood: No Mood
|
|
@Harristotle - Thank you for the information!
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by electrokinetic  | I recently received a rather large treasure trove of glassware from a friend who is a chem professor. Incredibly, he found all of it in or by the
dumpster when he was leaving work!!!
This piece is stuck to the top of a Vigreux column. My friend had no idea what it was other than the obvious: it was part of a distillation setup. I
have searched google, and posted it on another forum. Someone finally suggested that it is probably a distillation splash head, and I think he's
probably right, but I thought I'd post it here and see what the community here says. After all, as the someone also pointed out, if it is a splash
head it would be pretty pointless at the top of a fractionating column.
This looks to be a custom made high capacity drying tube.
[Edited on 1-13-2016 by zts16] |
|
|
|
Ruski
Harmless

Posts: 1
Registered: 27-4-2016
Member Is Offline
Mood: No Mood
|
|
In This video on Potassium Chlorate Electrolysis there is a particularly bizzare piece of glassware, any ideas what you would call this thing?
https://www.youtube.com/watch?v=9FyGP-zSpZo
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The first one is what is called a 'Beaker' 
The next one i would call an offset 4-neck head, although i just made that up.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That's a reaction vessel. I think the 4-neck thing is called a "reaction vessel head." I understand that reaction vessels are not uncommon in
industry; they certainly look easier to clean than boiling flasks.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I have some of the reactor heads with 3, 4, and 5 joints, the bottoms I don't have much of, so if anyone has the bottom but not the top, let me know.
They are basically jars with flanges, so that part might be cheaper, but few things in chemistry are reasonable.
|
|
|
jamit
Hazard to Others
  
Posts: 375
Registered: 18-6-2010
Location: Midwest USA
Member Is Offline
Mood: No Mood
|
|
I have the bottom and top of these reaction vessels. They are amazing! Way better in my opinion than rbf for distillation -- they look amazing.
Plus it is so much easier to clean. the key piece of equipment for these reaction vessel are the clamps. they are not cheap. around 100.00.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Found this today at my school. It has a fritted disc in the right chamber, and the tube connects the two chambers, but the vertical part at the botom
is just a solid piece of glass.

|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Interesting piece of glass. Let's see if we can figure it out.
The only plausible reason for the fritted glass that I can think of is for filtering. Long and thin so it must be a hassle to clean -- unless of
course it is only going to trap a very small amount of something. And of course it needs to be on suction. Left side has ground glass joint so
suction adapter can be put on easily.
I think it is a device for cleaning gases. Filter particulates out on one side. Dessicate it on the other. Actually looks pretty useful to me.
Short solid bit of glass probably fits into a holder of some kind -- either for use or for storage.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
http://www.ebay.com/itm/Ace-glass-soxhlet-apparatus-50-30-in...
Advertised as a soxhlet. Obviously it is not. But what is it?

And then there is this one:
www.ebay.com/itm/Kontes-glass-dual-column-evaporater-condens...

[Edited on 14-5-2016 by j_sum1]
|
|
|
Funkerman23
Hazard to Others
  
Posts: 416
Registered: 4-1-2012
Location: Dixie
Member Is Offline
Mood: No Mood
|
|
If this helps, its part of a sulfur determination apparatus. Here is a site that sells them ( supposedly) http://www.krackeler.com/catalog/product/3668/Corning-Pyrex-... . I'll have to look elsewhere for docs on how to actually use said apparatus
though, as I have never needed one nor have had the chance to use one.. Quote: Originally posted by zts16  | Found this today at my school. It has a fritted disc in the right chamber, and the tube connects the two chambers, but the vertical part at the botom
is just a solid piece of glass.
|
[Edited on 2-8-2016 by Funkerman23]
" the Modern Chemist is inundated with literature"-Unknown
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
I found this glassware in the place where I work. I asked everyone who worked in that lab, but noone has a clue on what it is and it looks like it
hasn't been used for like 15+ years.
    
It's a bit sad, considering it is in good condition (just a bit dusty). I'm somewhat certain it was used for some type of distillation.
I've drawn a sketch to show how the flasks are connected:
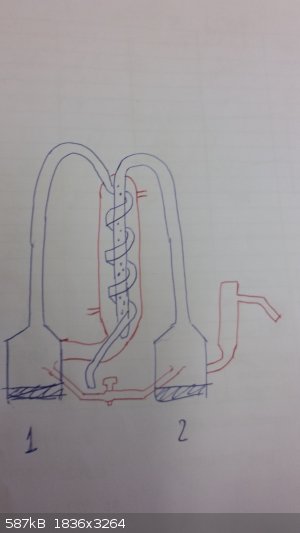
The first flask is connected to a serpentine and to what resembles a condenser. The second flask is connected to a pipe that dips in the "condenser"
and there are small holes on it (maybe to absorb/desorb gas?).
It got 2 heaters (caldaia), 1 cooling system (raff) and a "line" button that I don't know what is used for.
There is also a tube connecting the two columns which is regulated by a switch.
Any idea on what it is and how and why it was used?
I tried figuring out how it works but I'm stuck in a loop:
1) Let's assume you start filling the "condenser" from the bottom to the top with water; since it's connected to the 1st flask, by gravity it will
also inevitably fill the 1st flask up to the height of the top exit. If the filling rate is slow, the water will not be able to overflow and pass
through the serpentine. Start heating the 1st flask: if the water in the 1st flask was isolated, it would evaporate, pass through the serpentine,
condense on the cold water of the condenser and finally exit. However, since they are linked, I don't see the usefulness of doing such thing (you
would be heating water that gets replaced with cold water everytime and would not be able to evaporate. Even if the filling rate was so slow to allow
for the evaporation of water, you would have hot water in the 1st flask and in the "condenser", so why would you do such thing?).
2) Let's assume you fill the 1st flask to the height of the bottom entry, then stop. Start heating the 1st flask: the water will evaporate, pass
through the serpentine that dips in... hot water and then exit. Because the 1st flask and condenser are linked, the water will be hot in both, so I
don't really see the purpose of this.
3) Let's assume you don't fill the condenser and close the water entry/exit. Start heating the liquid inside the 2nd flask; this will evaporate and
pass through the pipe that dips in the condenser. If it condenses, it will end up in the 1st flask. Start heating the 1st flask, the freshly distilled
liquid will evaporate again and pass through the serpentine, "condense" (??) and exit. This way it would function as a multi-step distillation, but
then what is the purpose of the condenser? Couldn't it have been manifactured in a less complex manner if that was the purpose?
What really bugs me is the fact that the first flask is linked to the condenser.
PS: I wouldn't know how to disassemble it. Every piece is melted together, so there is no way to clean/scrub it from nasty stuff, if not passing tap
water through it. This led me to think it was mainly used with water or for degassing something not so nasty. Maybe to distill water? Don't know
[Edited on 4-8-2016 by Metallus]
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
it could just be a dH2O still,
water in at right, auto-fill of first pot (2) distill to pot1 for a second distillation ?
(assuming a glass disc sealing the bottom of the condenser)
The clip on the condenser could be something like a thermistor or thermal switch to remove heating power in case of loss of cooling water flow?
Use discontinued due to poor location of drain taps ?
[Edited on 4-8-2016 by Sulaiman]
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | it could just be a dH2O still,
water in at right, auto-fill of first pot (2) distill to pot2 for a second distillation ?
(assuming a glass disc sealing the bottom of the condenser)
The clip on the condenser could be something like a thermistor or thermal switch to remove heating power in case of loss of cooling water flow?
Use discontinued due to poor location of drain taps ?
[Edited on 4-8-2016 by Sulaiman] |
I went and checked. Indeed the bottom of the condenser is sealed. Damn, I looked at it from every angle and it didn't seem sealed at all. That was the
single thing that was irritating me. The fact that the condenser and the flask were connected didn't make sense at all to me. I had to actually fill
it water to see that it wouldn't go in the first flask.
Ok, with this setup now it makes sense:
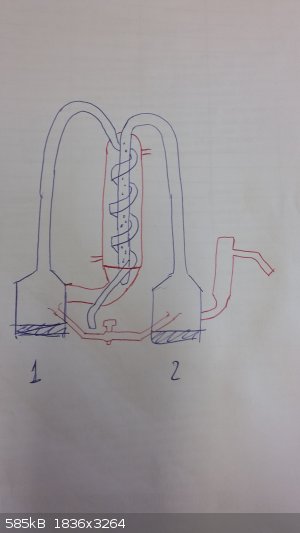
Boil water in the 2nd flask, it evaporates and condenses in the pipe that is isolated from the condenser (it doesn't actually dip in it as I feared
but goes through it). The condensed water enters the 1st flask where it boils again and condenses in the serpentine, and finally comes out.
Now I only have spare questions:
1) What are those small holes in the pipe for? I can clearly see these super tiny holes, maybe not big enough to let water through, but what are they
for?
2) Is there any particular reason as to why the first time it condenses it's through a straight pipe and the second time it condenses is thorugh a
serpentine?
3) Do you think this setup could be employed to distill something else outside water? I mean, something that may actually require a second
distillation, like crude oil, or a fuel or some alcohol etc. Which substance would benefit the most from this apparatus? We already have an automatic
machine for ultrapure distilled water and I wanted to give this apparatus a reason to exist. It looked cool the first time I saw it 
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
are they holes? (not clear on my laptop)
could be Vigreaux-style indentations to increase surface area
which would answer Q2 also.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | are they holes? (not clear on my laptop)
could be Vigreaux-style indentations to increase surface area
which would answer Q2 also. |
Yes, taking a close look to the pic I posted, they look like inward fingers, so they are most likely Vigreaux indendations as you said.
Do you know what use could have the pipe connecting the two flasks? Why would you ever connect them with the switch?
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
my guess would be a three way valve;
left/none/right connected to outlet, hole in tap is a 90 degree bend ?
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Today I confirmed that those indendations aren't exactly vigreaux ones (the latter have a 45° inclination, these are at 0°), but they are most
certainly used for the same reason.
As far as the valve is concerned, I found out that . ... it has no outlets lol. It's simply sitting there, with no holes, blocking the connection
between the two flasks no matter how you turn it. It is just a plug.
I don't know why they'd put it there. Maybe pull it out to empty omogeneously the two flasks? What would be the purpose of such thing though?
And assuming it was just a replacement for a valve that got lost, why would you manifacture said valve with a 90° outlet fashion? Do you think there
could be an application for a valve that actually connects the two flasks when switched on the "on" position?
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I would remove the drain tap for inspection as the hole (or groove) may be blocked with carbonates etc.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
I had already removed it for close inspection. It's a frosted tap, smooth and flat. I also inspected the bottom, which was transparent, to see if I
could spot an internal "canal" or something, but it is just a plug.
I'll go take a photo
-
With flash and without (I wetted it to see through the frosting)
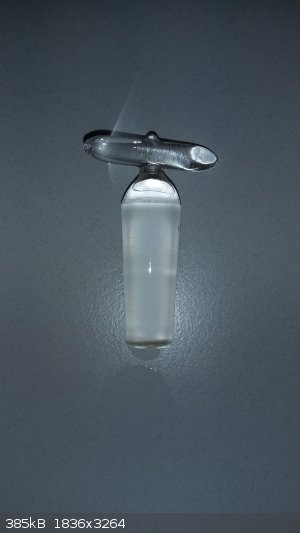 
[Edited on 5-8-2016 by Metallus]
|
|
|
Toady
Harmless

Posts: 15
Registered: 16-10-2016
Location: Beyond the pale
Member Is Offline
Mood: PISSED
|
|
Greetings.
Toady here, aka Tsathoggua, but you can call me Tsath'.
Tried to register under that nick, but it looks like it was already taken. Although I do not recollect having registered previously. Not surprising,
post excitotoxicity clusterfuck. May well not have been me, of course.
Anyway, first post. A mystery bit of glass, going for pocket change on ebay some time ago, snapped it up and figured I'll find out what it's for then
put it to good use.
Seller had no idea, it was part of a mixed collection of random items and it seems like they just happened to have a piece of lab glass knocking
around.
The top is a ground glass female fitting with a fairly long, curved hose barb about 1'' from the end of the ground glass joint proximal to the barb,
connecting to a bulb, a bump trap I'm thinking here, which has an upward curved short connecting section of glass tubing leading down into a male
joint, which widens just slightly at the beginning of the joint taper and above it, compared to the downward section, which also connects (this is the
secxtion on the left) to the wider bore section of glass connected to the bulb, situated above the left hand fitting on the lower end is another tube
connecting it with the central bore, U-bend shaped, whilst the right side bears another male joint of similar shape, with a doubly connected section
of tubing, the lower connection is situated just above the bottom of the central bore. Whilst the one connected by the U-bend on the left hand side
leads directly into the base.
Not seen anything like this before. Best I can think of is some kind of Dean-Stark/still bastard-child.

|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I'll agree that it is weird. Left side looks like the siphon on a soxhlet. Right side looks like Dean Stark -- or more particularly the type of
collection unit used in essential oil extractions. I can't really see how you would get the two to work together though.
|
|
|
Toady
Harmless

Posts: 15
Registered: 16-10-2016
Location: Beyond the pale
Member Is Offline
Mood: PISSED
|
|
Could it be for removing water or other solvent from a second solvent azeotropically whilst recirculating the solvent from which the second substance
(be it water or other solvent) is to be removed through either something like a soxhlet thimble or other filter of some kind?
Not familiar with the equipment concerning essential oil distillation you speak of, aside from the principles of steam distilllation. However the way
I would go about a steam distillation of course, is going to differ from how industry does it.
|
|
|
| Pages:
1
..
3
4
5
6
7
..
16 |