| Pages:
1
..
44
45
46
47
48
..
77 |
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
how wet was your ethanol? Also, doesn't the equilibrium of hydroxide and ethanol lie heavily to the product side because of the water generated and
the pKa's? The org syn procedure uses sodium metal. http://www.orgsyn.org/demo.aspx?prep=CV3P0016 Nurdrage used molecular sieves to make sodium methoxide from methanol and sodium hydroxide, so maybe
you could try that if you don't have sodium,
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by Hegi  | Quote: Originally posted by zts16  | Nice! I'm actually currently working on an indigo synthesis too, however I'm actually synthesizing my own 2-nitrobenzaldehyde, so it's a more lengthy
procedure. I have 2-nitrotoluene now, and I'm currently awaiting the arrival of the solvent that I need for the oxidation step. Then it should be
smooth sailing from there. A writeup will be posted once I am finished. 
|
Oh man, such a long way. The last step is really smooth sailing besides making your own 2-nitrobenzaldehyde. Did you prepare 2-nitrotoluene?
|
Yes, I prepared mixed nitrotoluenes by nitration of toluene, and then fractionally crystallized out the p
isomer by sticking it in the freezer, waiting for it to freeze out, filtering, and repeating the process again. I actually made use of the p-isomer
too, by making an azo compound, 4,4'-dimethylazobenzene, by reaction with zinc and sodium hydroxide in methanol under reflux. I have it as a solution
in DCM. It's not as pretty as I'd hoped: looks a bit like unhealthy urine.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Zephyr  | Here is the synthesis I attempted. As you can see moisture leads to issues, and I suspect it was the culprit... A bit of
Insight on how it could be done better or issues with this route are appreciated.
|
I think your scheme is wrong because it looks like you work with an alcoolate of 4-hydroxy-pentan-2-one.
2,4-Pentandione (CH3-CO-CH2-CO-CH3 equal to CH3-C(=O)-CH2-C(=O)-CH3 ) can make a stabler enol because of the resonance between the C=C double link and
the C=O double link...such systems are acidic see phenol.
CH3-C(-OH)=CH-C(=O)-CH3 <==> CH3-C(=O)-CH2-C(=O)-CH3 <==> CH3-C(=O)-CH=C(-OH)-CH3
So it looks like you simply have forgotten the C=C double link in your scheme and that the resonance hybrid includes the C atom between the two C=O
groups.
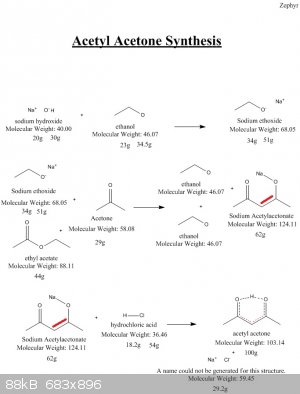
[Edited on 25-3-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Some Thallium Nitrate on an Iron nail in the flame, a beautiful green although its pretty hard to get on cam, nothing like Barium or Copper would
emit.
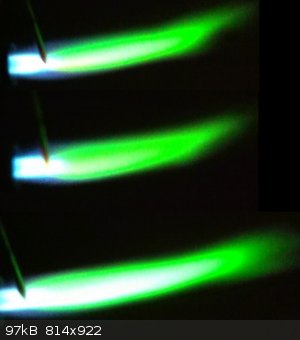
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice thallium photo! SM Wiki quality...
Zts16, I've been noticing recently that a lot of the solutions within my lab-work end up being urine colored in some way. Yellow is becoming one of my
favorite colors, though, along with green, blue and orange...
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
@TheVolatileChemist:
Thanks. Take it if you need it for the wiki. As our Cyanide Chemist I should probably do some pages on transition metal cyanides for our Wiki but I'm
too busy doing other stuff and I don't like writing articles  . .
Also if anyone knows a cool colored complex with Thallium I'd be interested. I have an Ebook on Thallium Chemistry but I won't buy special reagents
just for that single experiment. So no complicated organic ligands. I found 2 interesting, one with Chromium and Thallium(III)Chloride where Thallium
is really in a [TlCl6]-complex so not just a precipitate and one with Cobalt. Problem the fist one requires Hexamminechromium(III)chloride which is
either done using cold Sodium Amide or cold temperatures and quite a lot of Ammonia and Cr(II). So nothing I have time for at the moment. And for the
Cobalt one...well that was kinda my bad to accientally use Potassium Nitrite instead of Sodium Nitrite and I now have the precipitated Potassium salt
-.- . So if anyone has a good suggestion, I don't want to waste it since I'll be needing it soon but for one or two cool compounds I'd gladly
sacrifice some of it.
[Edited on 2-4-2016 by fluorescence]
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Nothing fancy, just some urea needle-shaped crystals I made a few days ago. Longest crystals are about 5 cm long.
Too bad they broke when I tried to get them out of the beaker (they are very fragile) 

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by Mabus  | Nothing fancy, just some urea needle-shaped crystals I made a few days ago. Longest crystals are about 5 cm long.
Too bad they broke when I tried to get them out of the beaker (they are very fragile) 
|
They look really amazing  Nice one Mabus! How fast did they grow? Nice one Mabus! How fast did they grow?
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by Hegi  | Quote: Originally posted by Mabus  | Nothing fancy, just some urea needle-shaped crystals I made a few days ago. Longest crystals are about 5 cm long.
Too bad they broke when I tried to get them out of the beaker (they are very fragile) 
|
They look really amazing  Nice one Mabus! How fast did they grow? Nice one Mabus! How fast did they grow?
|
I left them to grow overnight, between 12-18 hours. I think it also helped that the temperature slowly went down from 20 °C in the noon to 5 °C
during the night.
I didn't intend to grow such large crystals, I merely wanted to purify some urea I got for free.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by Mabus  | Quote: Originally posted by Hegi  | Quote: Originally posted by Mabus  | Nothing fancy, just some urea needle-shaped crystals I made a few days ago. Longest crystals are about 5 cm long.
Too bad they broke when I tried to get them out of the beaker (they are very fragile) 
|
They look really amazing  Nice one Mabus! How fast did they grow? Nice one Mabus! How fast did they grow?
|
I left them to grow overnight, between 12-18 hours. I think it also helped that the temperature slowly went down from 20 °C in the noon to 5 °C
during the night.
I didn't intend to grow such large crystals, I merely wanted to purify some urea I got for free. |
This slow decrease in temperature overnight is the goal. Really nice.
A picture of tridymite I found yesterday in a stone pit. The crystals are well shaped and up to 4 mm in lenght.
<img src="http://chem.pieceofscience.com/wp-content/uploads/2016/04/tridymit1.png" alt="Mountain View" style="width:900px;height:600px;">
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Just wanted to say WOW !
I spent quite a while looking at each, so much going on.
I especially liked the soot generating flame, sort of fills in a mental picture.
more or a pointer to more would be nice.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
So....I have no idea what I just did.
Some time ago a friend gave me a bag full of natural Arsenic, he purchsed that somewhere, I dunno. Some black chunks, some have impurities some are
quite pure. I did some experiments with them like dissolving them, there is a pictrue in this thread. So I decided to try to make some Oxide from it
by heating it. I took some small chunks into the test tube and heated them with the burner till the glass became quite soft, dunno where the melting
point of my glass is. The chuncks started to smoke producing a dense brown smoke. I stopped and the thing you see on the picture formed. I than took
the test tube and turned it upside down. The Arsenic fell out and started smoking brown again. You can see a dark brown trail on the upper part of the
test tube where they touched the glass when falling out.
That is not what I expected. I tried this before, back then with a heat gun so it was much slower and back then only white stuff had formed.
Ehm...well it looks like an Arsenic mirror, its fully reflective, as you can see there is my reflection in it its silvery on the outside and on the
inside it has a quite rough black surface.
It really reminds me of the Arsenic you make using the Marsh test. Also I noticed a familiar smell comiing from the test tube after it cooled down. It
reminds me of the smell that Phosphorus has when you burn it. It wil alwys leave behind a white, waxy rest when its burned and that usually smells
like the stuff coming from the test tube....
Anny suggestions ? Maybe Sulfide or something that fell apart under the hot temperatures forming elemental Arsenic ?
I read about a suboxide of Arsenic that can form and that smells like phosphorus and is brown as well.
I mean I could have distilled Arsenic as well although I'm not sure if the temperatures really reached 600°C and maybe the fine powdered arsenic when
cooled down got coated with this suboxide that appearently smells like garlic. That could be an explanation.
Hard to admit for me as an Inorganic Chemist but I have no idea what this could really be.

Edit:
I took one of the small grains and heated it directly with the bunsen burner on a metal surface. It immediately formed the same black stuff behind the
metal. So I guess that by heating it wih the burner in the test tube it got the sublimation point of Arsenic faster than Arsenic reacts at these
temperatures with the oxygen to form the Oxide. So I guess the black stuff it just Arsenic and some small amounts of Oxides which give the smell.
[Edited on 9-4-2016 by fluorescence]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Native arsenic is surpringly common, while not widespread, when it does occur it is often abundant. Over my life time in the mining industry I have
acquired many incredible specimens some several kgs in weight. However, it is often alloyed with native antimony to form AsSb or stibarsen.
Your last point is almost certainly correct. When strongly heated arsenic sublimes in the absence of air about otherwise it oxidizes to white arsenic
As2O3 though in a test tube much may sublime as insufficient air can get in quickly.
If you want to make something out of it: nitric acid is an easier way to oxidize it, it gives arsenic acid (and much brown N2O4)and any antimony
impurity is oxidized to sparingly soluble Sb2O3. Arsenic dissolves in alkaline oxidizing solution (ie bleach) as sodium arsenite and in polysulphide
solution to give various thioarsenates. Melting with sulphur generates various sulphids depending on ratios and these dissolve in hydroxide, alkaline
sulphide and polysulphide solutions to give thioarsenites and thioarsenates. Hydrogen peroxides converts thioarsenates to arsenates.
|
|
|
100PercentChemistry
Hazard to Others
  
Posts: 117
Registered: 21-8-2015
Location: On the island of stability
Member Is Offline
Mood: No Mood
|
|
Tin crystals

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
@Boffs: Thank you for the ideas. I tried to make Arsenic Acids but I avoid that topic or it gives me headaches otherwise. Problem is that literature
isnt very precise on what actiually forms at what moment. And I think you need to really heat the Arsenous Acid to get to the pure Arsenic Acid or
something like that. At least its not easy to get a pure form of one of the two and some books just confuse that too much. So I dont even start to
untangle that. But there are some interesting As Compounds and I will try to cover them as well. I'm still stuck with Tl at the moment but after that
I will try to find some interesting As compounds and hopefully post some good pictures here. Any suggestions are welcome.
[Edited on 11-4-2016 by fluorescence]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@fluorescence check out; Handbook of Preparative Inorganic Chemistry by Brauer on the forum library.
More information is available in the appropriate volumes of A Textbook of Inorganic Chemistry (try volume VI part 4) and A Comprehensive Treatise on
Inorganic and Theoretical Chemistry by Mellor, both of which are also on the forum's library page.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Here is some Thallium Chemistry. I wasn't that creative this time simply because I didn't have too much time. Still I like the results. Those are only
very small amounts due to the fact that pure Thallium Salts are really expensive and I need them for some other experiments soon and they are not that
well soluble as you might expect at least if you dont boil the solution. So I stayed with small samples and still enough compounds formed. Note that
all of these compounds are insoluble and seperated pretty fast I just shook them before I took the photos so you could see them better.
I really like how the colors vary with Potassium Ferrocyanide and Chromate both being yellow and many other prec. Ferrocyanides being brownish as well
it surprises how the Thallium sample can form a white compound and a yellow one. Usually I check these reactions before I post them here but I didnt
have time so if there is any mistake like a side reaction I didnt think of just tell me.
To the Selenide. I suppose this is the Selenide. I tried a new method to make transition metal chalcogenides. There will be a post on it soon i am
still drying the Cadmium Sulfide I made for test and then I have to test if its really the Sulfide and not something else. After that I will make a
post on how I made this Selenide.

|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Interesting work fluorescence. How confident are you in your identification of these substances?
Are you certain that you have no III oxidation state substances there?
I too am suspicious of the selenide. It looks like you might have something else suspended in there.
J.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
As said I don't know. Some experiments need to be done with Tl(III) which is sometimes made in a Chlorate + HCl boling mixture so this needs a lot
power. But I could just check all these structres. As Solid State Chemist I have acess to all the Crystall Structure Data:
So let's check it:
- For the Thallium(I)Selenide, I4/mcm there are some papers on it and none is on Tl(III). I just assume that crystals structures imply that at least
someone extracted a pure sample and calculated if the structure can form with the given Atoms and ratio, at least this is what I have to do with my
solids.
I checked Reaxys for the preparation, some literature suggests melting the pure Elements together, and some make it from a Selenium Source and a
soluble Thallium Salt in solution. And since the SUlfide precipitates as well I assume that it's possible with the Selenide as well. And most sources
describe it as black like my stuff is.
- For the Chromate, yeah it appears like Chomate could perhaps oxidize it to Tl(III) somehow. Indeed under acidic conditions Dichromate would be a bit
stronger with its redox potential but that would require an acid in there as well.
So lets check the crystal database and the result is there are the following known structures
o Thallium(I) Chromate -> Pmcn/Pnma
o Thallium(I) Dichromate -> P-1
and indeed there is a Thallium (III) Chromate -> Pbcn mentioned.
So let's first check out the paper that is given for the Tl(III):
Too bad I can't find any digital version of it but there is something in the Pigment Compendium. And it seems like the color varies from yellow to
red depending if Chromate or Dichromate is used and Tl(I) or Tl(III) is present. Reaxys suggests simple precipitation for the Tl(I) salts and for
Tl(III) there is only one descirption where Tl(Iii) Nitrate is reacted with Chromium(VI)Oxide over two days at 40°C.
o Next would be Thallium Thiocyanate:
There is only a Tl(I) known with a room temperature structure of Pbcm (like KSCN) and a high temperatue phase of I4/mcm. I think potassium does the
same thing with two different structures. Sodium in contrary has only one structure at least I never found a high temperature NaSCN phase in the
literature. So Tl(I) seems to imitate Alkali Metals here and if its insoluble it might be stable as well. According to Reaxys it forms with
Thiocyanates and Thallium(I) solutions. And I saw that all solubilities in literature are quite low so I suppose it should precipiate as well.
o For the Ferrocyanide:
I cant find the paper at the moment but I found one earlier. I can only find crystal data on the one with Tl(I) and Fe(II) [P-1]. Interesting thing is
the paper said that the crystals are actually yellowish and my stuff is white but they said that it was hard to actually make crytsals and upon
touching them they would fall apart to a fine powder. I guess mine is just so small that it scatters light differently and appears white. But I will
check the other databases tomorrow and see if I can find more information on the Thiocyanate as well.
But it appears that my ideas where correct. Any suggestions, corrections welcome ! I will check the Gmelin on that if I find some time tomorrow.
[Edited on 25-4-2016 by fluorescence]
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Addtional:
I found something on the ferrocyanide. According to Alfred Brukl - Elemente der dritten Hauptgruppe Band II,
Thallium(I) can be oxidized to Thallium(III) using either bromine water in acidic conditions or Potassium Ferricyanide so with Fe(III) in there under
alkaline conditions. I will try that out when I have more time, seems quite interesting. It says here that Thallium(III)Hydroxide is precipitated and
the yellow Fe(II)-Ferrocyanide can be titrated with Permanganate to tell how much Thallium there actually was.
I will try both methods soon, so Tl(I) to Tl(III) with Br2 and with Ferricyanide and I'll try to make some other Tl(III) compounds as well.
[Edited on 27-4-2016 by fluorescence]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
OH YEAH!!! Sassafras Albidum (it IS chemistry related, mua-ha-ha)

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
Arkoma, binomial names have the species part written in all lowercase! 
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That stuff is pretty common. They are most famous for their root bark essential oil, but I am curious about why the leaves smell like lemons.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
The root bark oil is Safrole--Yee-Ha. Just spotted probably another 50 trees on the side of the back road.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Deathunter88
National Hazard
   
Posts: 522
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Barium chloride crystallising out of solution!
Largest crystal was about 15cm long, smaller crystals around 3cm ish. It happened by accident as I was evaporating down a solution of barium chloride
I had just made with HCl and barium carbonate. I managed to keep most of them intact when getting them out of the dish.

|
|
|
| Pages:
1
..
44
45
46
47
48
..
77 |