urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
UO2 looking for ways to react it
Hey forum! So I have 7 grams of crude, natural uranium dioxide and Im looking for ways to react this to get different uranium compounds. Ive tried HCl
and it doesnt do anything at all. Is there any way to get that pesky oxygen off of the uranium so I can do other chemistry with it?
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I recall a shocking YT video in the thread on bad youtube videos. From memory it clocks in at about post 40-50. I will look it up for you.
I would not emulate the procedure but it may be that the theory is worth a look.
I am sure that someone more knowledgeable will be here to point you in the right direction. But in the meantime, you may as well be entertained /
shocked / informed by someone doing uranium chemistry beside the swimming pool.
Edit
Here is the thread and the relevant post. Thanks blogfast.
http://www.sciencemadness.org/talk/viewthread.php?tid=48892&...
[Edited on 4-4-2016 by j_sum1]
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Someone named Carl Willis has a very nice page with some practical uranium chemistry:
https://carlwillis.wordpress.com/2008/02/20/uranium-chemistr...
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Joe Skulan
Harmless

Posts: 21
Registered: 16-3-2016
Member Is Offline
Mood: No Mood
|
|
It will dissolve in nitric acid.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Apparently HCl and an oxidizer, bleach, conc. peroxide will work also.
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | I recall a shocking YT video in the thread on bad youtube videos. From memory it clocks in at about post 40-50. I will look it up for you.
I would not emulate the procedure but it may be that the theory is worth a look.
I am sure that someone more knowledgeable will be here to point you in the right direction. But in the meantime, you may as well be entertained /
shocked / informed by someone doing uranium chemistry beside the swimming pool.
Edit
Here is the thread and the relevant post. Thanks blogfast.
http://www.sciencemadness.org/talk/viewthread.php?tid=48892&...
[Edited on 4-4-2016 by j_sum1] |
Thanks for that video, I dont think that process will work with what I have though. Someone else here is suggesting nitric acid, I think Ill try that
before anything else. Thanks so much for the video, it was informative and the next time I find myself in the US southwest Ill try and get some of
that ore.
My UO2 is hard and glassy and came from veins of calcite at a road cut.
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Its certainly worth a try, Worst case is that it does nothing, best case Ill have uranyl chloride as a building block for more complex arrangements in
the future. Thanks.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Read the link phlogiston kindly provided. Very interesting chemistry.
Calcite, carbonates. You really need to read phlogiston's link.
[Edited on 5-4-2016 by hyfalcon]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Some rust remover contain HF and oxalic acid. It can also be used to obtain the tetrafluoride which precipitate out as a green solid
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by neptunium  | Some rust remover contain HF and oxalic acid. It can also be used to obtain the tetrafluoride which precipitate out as a green solid
|
Actually HF is one of the easier acids to make. CaF when treated with 98%H2SO4 releases relatively pure HF. The problem with pure HF is temperature.
It has to remain below 19oC or it vopourizes and puts huge stresses on the container it is in. I wasnt really interested in a uranium fluoride as its
difficult to do anything with it. I am currently reacting it in a strong solution of NaOH, and its slowly but surely converting to UO2(OH)2. From
there Ill see about making organic complexes from the hydroxide.
|
|
|
bur
Harmless

Posts: 4
Registered: 21-6-2016
Member Is Offline
Mood: No Mood
|
|
I know the tread is a bit old, but for the record, in my experience no oxidizer is required to dissolve U metal in hot nitric acid. So the same should
be true for UO2.
By the way, for purification the PUREX method could be interesting. Tributylphosphate very selectively complexes U, causing it to leave the water
layer. It's the commercial way to recover U and Pu from used fuel. I haven't tried it yet but it seems to be easy and efficient.
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bur  | I know the tread is a bit old, but for the record, in my experience no oxidizer is required to dissolve U metal in hot nitric acid. So the same should
be true for UO2.
By the way, for purification the PUREX method could be interesting. Tributylphosphate very selectively complexes U, causing it to leave the water
layer. It's the commercial way to recover U and Pu from used fuel. I haven't tried it yet but it seems to be easy and efficient.
|
Cold nitric acid (fuming) did the trick, now im just waiting for evaporation to get rid of the water. Thanks for the tip, it worked.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Maybe not what you're going for, but uranyl nitrate has some potential in photochemical transformations of alkanes (!).
| Quote: | | One aspect of uranium chemistry that has been well-studied, however, is the photochemistry of the uranyl cation (UO22+).[22-24] Studies by multiple
groups have revealed that this cation possesses several intriguing characteristics. Notably, a highly oxidizing excited state, [UO2]2+* (+2.6 V vs.
SHE, almost equal to the oxidizing power of elemental fluorine![25]), is accessible under blue light (hν 450–495 nm) irradiation. This excited
state is sufficiently reactive to abstract hydrogen atoms from unactivated C−H bonds (BDE >100 kcal mol−1) to generate carbon-centered
radicals. Furthermore, pioneering studies by Bakac and co-workers showed that this reactivity could be rendered catalytic for aerobic oxidation of
alkanes (Figure 2); with some substrates, the quantum yield approaches unity.[26, 27] Despite this promising reactivity and abundance of desirable
characteristics, the applications of the uranyl cation in catalysis remain largely underdeveloped. |
http://onlinelibrary.wiley.com/doi/10.1002/anie.201603149/fu...
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
Yamato71
Hazard to Self
 
Posts: 70
Registered: 8-2-2012
Member Is Offline
Mood: Resigned
|
|
When prospecting for uranium containing rare earth minerals at night in Texas, I carry an ultraviolet lamp and a spray bottle filled with either a 50%
solution of glacial acetic acid or 10% nitric acid in water. Almost all uranium specimens here are oxides, which are not fluorescent. By spraying
the diluted acid on a pitchblende or other specimen, the acid converts the outer layer of oxide to either the nitrate or the acetate, both of which
are fluorescent because they contain the uranyl (UO2)+2 ion in aqueous solution. After a minute or two, the UV lamp lights them up a nice bright
green.
|
|
|
Chlorine
Hazard to Self
 
Posts: 56
Registered: 26-11-2016
Location: Maine, USA
Member Is Offline
Mood: Brominated
|
|
It should dissolve in an HCL+HNO3 (3:1) solution.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Yamato71  | | When prospecting for uranium containing rare earth minerals at night in Texas, I carry an ultraviolet lamp and a spray bottle filled with either a 50%
solution of glacial acetic acid or 10% nitric acid in water. Almost all uranium specimens here are oxides, which are not fluorescent. By spraying
the diluted acid on a pitchblende or other specimen, the acid converts the outer layer of oxide to either the nitrate or the acetate, both of which
are fluorescent because they contain the uranyl (UO2)+2 ion in aqueous solution. After a minute or two, the UV lamp lights them up a nice bright
green. |
That... is actually pretty clever. Congrats, I'm definitely using that method when I go uranium prospecting.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I was thinking the exact same thing.
|
|
|
Radium212
Hazard to Self
 
Posts: 85
Registered: 26-6-2017
Member Is Offline
Mood: Pyrophoric.
|
|
I also recommend Carl Willis's procedure.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
| Quote: | Originally posted to Quora, written by Carl Willis
Uranium gets a bad rap, being widely regarded as exotic and dangerous. As a fellow "amateur chemist" (more recently, career nuclear engineer)
experienced with uranium, I would like to challenge this unfairly negative reputation and suggest that you CAN study the "household chemistry" of
uranium safely and responsibly without resorting to a poor surrogate. A basic working knowledge of inorganic chemistry is a prerequisite to a
satisfying experience; that is true of all chemistry. So be comfortable with stoichiometry, balancing equations, measuring mass and volume, etc. Be
comfortable with prevailing standards of chemical hygiene and safety, like wearing gloves and eye protection. The big dangers here are the same
mundane ones you'd find in high-school intro chemistry: strong acids and bases, hotplates, flammable solvents. Although a toxic heavy metal, uranium
is far more approachable than, say, arsenic or mercury or fluorine chemistry in a residential setting. The keys to good hygiene and responsible use
lie in working with small quantities (think about containing the mess if a container were to break or fall over) and controlling waste generation. Buy
some microscale glassware. Just be aware that if you work with large (~kg) quantities, or plan to sell or transfer things you make to other people,
you are no longer an inconsequential "hobbyist" and will find yourself up against the strictures of your jurisdiction's nuclear regulatory framework,
which in the USA is the domain of the Nuclear Regulatory Commission. The NRC defines, and subjects to a general license for use, "small quantities of
source material" below 1.5 kg; see 10 CFR 40.22. So if you play around with some introductory uranium chemistry, start small (with a couple grams of
material) to avoid the prospect of a soul-crushing run-in with regulators.
How to obtain uranium: You have two choices. First choice is to buy uranium on eBay, where there are regular sellers of compounds like uranyl nitrate
and oxide. Depending on your focus, you might be interested to know if your uranium source is "depleted" (low in the U-235 isotope) or "natural"
(0.72% U-235). Typically, commercial uranium compounds are made from depleted uranium (cheaply available from the nuclear fuel industry), while those
prepared from uranium ore directly are natural. Your second choice is to refine uranium from a concentrated ore specimen. This process is not
difficult (see my website link below), but it does generate a lot of waste that can't be put down your drain.
Basic chemical overview: Aqueous uranium chemistry is dominated by the uranyl ion (UO2)2+, with U in the U(VI) oxidation state. This is a
brightly-colored, greenish-yellow, UV-fluorescent species. Uranyl compounds are generally soluble in acids, and precipitate as marginally-soluble
yellow to orange uranate and diuranate salts with alkalis. Uranium in its lower (IV) oxidation state is sometimes encountered in the form of the
dioxide UO2 and the green tetrafluoride, UF4. These compounds are not appreciably water-soluble and in order to dissolve uranium in these forms, it
must be oxidized to (VI) with an appropriate reagent. Uranium metal is very hard to prepare in small quantities and you will probably not get an
opportunity to buy it in metallic form either.
Suggestions for chemistry of interest: Uranium is unusual chemically in a few respects. Consider its nearly-insoluble peroxide: no other
naturally-occurring elements have an insoluble peroxide at acid pH. Uranium forms some commercially-interesting complexes, like the uranyltricarbonate
anion (which is soluble in basic solutions, in which most transition metals precipitate) and the uranyl nitrate-tributyl phosphate complex (which
forms the basis of liquid-liquid solvent extraction of U and Pu in nuclear fuel reprocessing), and a peroxo complex that is vivid blood-red. You can
play with this chemistry at home because the other chemicals needed are ordinary. TBP has to be ordered from a chemical supplier, but you shouldn't be
given trouble for it. Uranium offers interesting opportunities for radiochemistry. Prepared uranium--depleted or natural--quickly builds up an
equilibrium concentration of U-238 decay daughters Th-234 and Pa-234. These are beta- and gamma-emitting nuclides, whereas the parent U-238 only emits
alpha particles. Thus, it is fun to see in any given chemical experiment with uranium if the apparent activity (on a common beta-gamma sensitive
Geiger counter) follows the uranium itself or not. Freshly-purified uranium compounds will seem minimally radioactive, but after a couple months Th
and Pa are back in the uranium! The waste from re-purifying daughter-contaminated uranium compounds may be left around to decay, and will exhibit the
easily-measurable half life about about three weeks due to the Th-234.
My own background: see my blog posts about household uranium chemistry here: http://carlwillis.wordpress.com/...
Dabbling in uranium chemistry is fun for the whole family, educational, and it ain't gonna kill you or the neighborhood as long as common sense
prevails.
Below: uranium compounds made by safe and responsible household techniques. See website for details.
Posted by Carol Willis on Quora.com |
(Edited to make clear that the majority of this post was cut and pasted from elsewhere)
[Edited on 5-12-2017 by Bert]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Maybe in the USA it is possible to obtain uranium compounds fairly easily. Where I live, it is really really difficult to obtain uranium compounds. I
was very lucky to get a sample of a few grams of uranyl nitrate in the form of some crystalline lumps. I do not use that for experiments (it is a too
small amount), I put it in a vial and use it as nice looking sample. In the Netherlands (and probably in the entire EU) it is not wise to tell that
you are doing experiments with uranium at home. I am quite sure that you will have a visit and that a hazmat team will take away all your nice uranium
stuff.
A few years ago I was given a few grams of finely ground pitchblende (a dark green ore, insoluble in water, having formula U3O8, I do not know whether
it is natural or artificial/depleted). I dissolved a small spatula of this in nitric acid and did a little experimenting with it (I obtained a lime
green solution and I made the pale yellow insoluble peroxo complex UO4.2H2O), but this was very small scale. Doing lots of experiments with uranium
simply cannot be safely done in the Netherlands.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
Dabbling in uranium chemistry is fun for the whole family, educational, and it ain't gonna kill you or the neighborhood as long as common sense
prevails.
|
Sense ain't common.
Carl Willis mentions degrees in both chemistry and nuclear engineering. Apparently, Carl also works in these fields professionally.
I would expect that certain things which appear to be common sense for Carl might not be so obvious to some others without his background?
Carl, if you happen to stop by here?
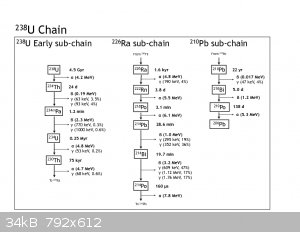
Could you please describe a safe, effective and responsible amateur scheme for storage and handling of lab wastes and radioactive residues? Also your
thoughts on a home experimenter concentrating or releasing other radioactive substances found in natural Uranium ore. The ones Mmme. Currie
occasionaly walked around with in her lab coat pocket, you know?
Extra points if the amateur handling scheme for nuclear materials and radioactive waste meets the letter of USA legal requirements, rather than being
judged to be all right by yourself as "too small scale to matter".
Believe me, I am all too familiar with the variance possible between being sensible/safe and being legal.
(Edited by Bert on noticing that the post by VSEPR_VOID was a cut & paste of someone else's work, thinking that authorship needed to be stated
more obviously)
[Edited on 5-12-2017 by Bert]
[Edited on 5-12-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
VSEPR_VOID, you might want to be more clear next time when you post something that isn't yours. At least enclose it in quote blocks and link to where
you copy/pasted it from. The footnote at the end about it being posted on Quora is easily missed and isn't a great way to attribute something.
Here is the original source: https://www.quora.com/I-would-like-to-study-the-chemistry-of...
|
|
|