rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
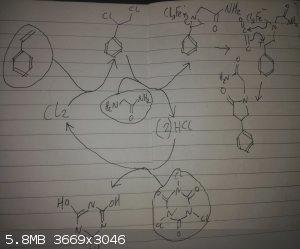
Possible 2 step synthesis of phenylpiracetam using OTC reagents
Hey there, I'm currently pursuing a professional chemistry career which I imagine prevents me from trying this synthesis myself because of the new
blanket banning of all psychotropic substances in the UK, still I find it fun to create theoretical syntheses for compounds,
Step 1:
Catalytic HCl
1 eq styrene (easily available through thermolysis of polystyrene)
1 eq glycine amide (dehydration of glycine with ammonia)
1/3 eq TCICA (available as a pool cleaner)
In DCM at low temperature ~0 degrees celcious
Step 2:
Ketene bubbled through solution (thermolysis of acetone in a ketene lamp)
TCICA reacts with an initial catalytic amount of HCl to generate chlorine, chlorine adds to double bond in styrene, low temperatures favours the
addition of 1,2-dihaloalkanes to an amine forming the aziridine, the solution of TCICA can be separate to the main reaction with a recycled gas flow
through the 2 flasks as HCl is not soluble in DCM, if there is a worry of side reactions,
the ketene part is the iffy bit, the Lewis acid was added as a hunch from other similar reactions I've read about, the aim is to weakly activate the
aziridine to form the appropriate zwitter ion, and hopefully the oxygen of the ketene will coordinate, allowing the alkene and the zwitter ion undergo
cycloaddition, (I worry about the C=O bond reacting instead from the strong dipole), I imagine different Lewis acids will give different yields by the
balancing of these properties,
I know ketene is 10 times more toxic than cyanide compatible to phosgene, but it is readily available from acetone, i would be interested in other
people's view on its viability, and strongly recommend nobody tries it as aziridines, HCl, Cl2, ketene are all very dangerous and can be fatal on
their own, plus the legal issues in some countries, just theoretical discussion please
|
|
|
Nicodem
|
Thread Moved
9-3-2016 at 11:33 |
Optimum
Harmless

Posts: 20
Registered: 4-8-2019
Member Is Offline
|
|
what are OTC reagents?
where you get these OTC reagents?
Thanks
[Edited on 4-8-2019 by Optimum]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I think it would be synthesised much easier and much more otc from phenibut.
According to wikipedia:
"Phenylpiracetam is readily-synthesized commercially. To avoid the problem of attaching the phenyl group to piracetam at position 5 rather than 3 or
4, commercial syntheses cyclizes phenibut by replacing the hydroxide with a hydrogen to create a pyrrole group and then reacting bromoacetic acid and
ammonia to replace the bromine with the nitrogen on the pyrrole. This produces a racemic phenylpiracetam, which then undergoes purification to produce
the final product."
[Edited on 8-8-2019 by mackolol]
|
|
|
|