| Pages:
1
2 |
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Manganese Mediated Electrochemical Oxidation of Ethanol
1. Procedure
The electrolyte is prepared by dissolving 64g MnSO₄*H₂O, 30g (NH₄)₂SO₄, and 193ml 17.5M H₂SO₄(aq) in 100ml H₂O. This electrolyte is
loaded into a three necked flask which is attached to a distillation rig. The remaining 2 joints are used for attaching two lead electrodes, each with
a surface area of approximately 1400mm². Next, 19V @ 4.7A is run through the electrodes and the reactor is run for 30min to ensure that all
components function properly. During this time, a purple color is observed in the electrolyte. Following this, 100ml 12.0M ethanol is than added to
the reactor. The reactor is run at 60*C until no more distillate condenses in the receiver. Note that these steps may be repeated as many times as
desired due to the electrochemical regeneration off the oxidizing agent.

An image of the purple color observed.

An image of the apparatus. On the left is a PID used to maintain the temperature at 60*C.
2. Notes
This procedure can be greatly improved by utilizing a more efficient method of stirring. A magnetic stir bar was used in this procedure, but it
quickly became useless due to the formation of a white precipitate in the bottom of the flask. A similar procedure was attempted utilizing Cerium
instead of Manganese, but this reaction failed because the ethanol was over oxidized to ethanoic acid.
3. Discussion
4. References
1.Sciencemadness Forum Thread (http://www.sciencemadness.org/talk/viewthread.php?tid=6882)
2.US Patent 80895 (http://www.google.com/patents/US808095.)
[Edited on 2-19-2016 by nlegaux]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
What was the (approximate) surface area of the electrodes you used? (Also, your image links don't seem to be working.)
I would appreciate more characterization of your acetaldehyde fraction (b.p. and density). The odor of acetaldehyde is by itself not a very good
indicator of purity.
Besides toluene and ethanol, I wonder if these oxidation conditions can be used on other substrates.
Having personally done this with toluene, I can agree that a strong stirring mechanism is necessary. A quite large magnetic stir bar was sufficient
for me, but mechanical stirring is likely the best option.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This doesn't belong in prepublication, IMHO.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Comments like this don't belong in prepublication, IMHO.
It's called prepublication because it is not yet published though the intention is for it to be eventually. If you don't think it's at that level yet,
why not offer some advice or constructive criticism? Or if you don't have anything to add to it, just don't say anything...
I think that it's an interesting and potentially quite useful procedure. The write-up could use some better formatting and more details in general.
Also, it is necessary to undertake better characterization of the product (at least a BP), and possibly a workup procedure to purify it since it is
discolored and likely also contains some unreacted ethanol.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  |
I think that it's an interesting and potentially quite useful procedure. The write-up could use some better formatting and more details in general.
Also, it is necessary to undertake better characterization of the product (at least a BP), and possibly a workup procedure to purify it since it is
discolored and likely also contains some unreacted ethanol. |
So do I. But it adds very little to the procedure already described in the first link, which is where I do honestly believe this work would be an
interesting addition. Furthermore only one in three links work and there's no product characterisation whatsoever.
So I stand by what I wrote: interesting work by all means but no need to go into prepublication.
[Edited on 17-2-2016 by blogfast25]
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Link No 1 & 3 worked for me.
As far as 2nd link is concerned, knowing the US patent number is enough.
For uninitiated the patent is attached.
gsd
Attachment: US808095.pdf (322kB)
This file has been downloaded 521 times
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
I should be able to begin work on further characterizing the product tomorrow. As far as organization goes, what is suggested? I haven't seen any
clear outline of what should be done.
Thank you,
nlegaux
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
@nlegaux
AFAIK there are no clear cut written guidelines for Pre-pub, nor is the format specified.
However I would suggest that you take a look at posts made in that section. All well written posts follow a certain format and syntax which makes them
extremely readable.
I would especially recommend any post made there by magpie as a template to follow.
You are working on a very interesting project and it would be very nice to see it is presented in structured and organised way.
gsd
[Edited on 18-2-2016 by gsd]
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
This is a cool project given the obnoxiousness of preparing acetaldehyde by other means (jones oxidation for example). I think you need to fractionate
the distillate to remove any ethanol that co-distilled and then give us a weight of pure acetaldehyde obtained.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@nlegaux:
Assuming you have these photos on your HD you can upload them to SM and show them directly in your post. In Preview Post mode, choose the file from
your HD, then click Preview Post again. The file will appear at the bottom as 'file ####', cut and paste this into the body of your post, where you
want the picture to appear. No need for imgur.
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Thank you for the advice blogfast! I updated the procedure with embedded photos. I will take a closer look at the formatting of some of the other
posts in prepublication and update this one ASAP.
I went ahead and began a workup. I recorded the boiling point at 23.4*C (lit. 20.2*C), but the amount of product that distilled is too small for me to
accurately obtain a density for. I will probably rerun this procedure this weekend. I believe adding the ethanol dropwise rather than in batches may
increase yields by giving the oxidant more time to regenerate (now just to find a way to add an addition funnel and still have the apparatus fit into
my small fumehood  ). ).
nlegaux
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@nlegaux:
Much better already.
Do you have any idea what the white precipitate is? Lead sulphate seems like a reasonable guess, in which case a different electrode material may be
worth considering. I see nothing in the Patent that explains lead is mandatory...
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
I'm not sure, but when I rerun it this weekend I will collect some and attempt some characterization. If it does turn out to be a lead salt, I will
investigate alternative electrode materials.
nlegaux
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I think the ability of Mn(+3) to oxidise alcohols to aldehydes (but not to carboxylic acids):
R-CH2-OH + 2H2O ===> R-CHO + 2 H3O<sup>+</sup> + 2 e
2 x [Mn<sup>3+</sup> + e ===> Mn<sup>2+</sup>]
... might also be used without any electrolysis.
In this rather long thread, woelen, me and Xenoid established that Mn(+3) can also be prepared via another method:
http://www.sciencemadness.org/talk/viewthread.php?tid=11309&...
MnO2 is reacted with 98 % H2SO4 to form a green and insoluble mangani - sulfato complex. On dilution with
water the complex dissolves and forms a solution of Mn2(SO4)3. Excess acid used in the first step stabilises it.
Adding the equivalent amount of alcohol should then result in its oxidation to aldehyde and would be distilled off.
The manganous sulphate could be recovered by precipitation as Mn(OH)2 and re-oxidised by air to MnO2.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Another quite interesting possibility was explored by ChemPlayer: the oxidation of benzyl alcohol with 'fresh' MnO2 in DCM solvent:
https://www.youtube.com/watch?v=HrZAMlj0kPM
Starts at about 4:31 (first part is preparation of MnO2).
Presumed reactions:
Ph-CH2-OH ===> Ph-CHO + 2 H<sup>+</sup> + 2 e
2 MnO2 + 2 H<sup>+</sup> + 2 e ===> Mn2O3 + H2O
I wonder if such a reaction would work for EtOH, perhaps even w/o DCM and with bit of conc. H2SO4 thrown in as presumed
catalyst.
[Edited on 19-2-2016 by blogfast25]
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
That is quite interesting. I once attempted using MnO₂ to oxidize 4-aminophenol (from hydrolyzed Pararcetamol) to benzoquinone (similarly to the
prepchem procedure found here http://www.prepchem.com/synthesis-of-p-benzoquinone/). Instead of benzoquinone, however, all I got was a terrible sticky black residue to clean
out of my flask.
I did a bit of work with the cell yesterday, and in the process I corroded one of my electrodes to a point that it is no longer useful. I think I will
make a few more lead electrodes for experimentation in the immediate future, but I will definitely need to investigate more corrosion-resistant
electrode materials (perhaps platinated titanium?).
Also, would it be more appropriate to move this thread to organic chemistry or technochemistry until I have created a finalized procedure?
nlegaux
[Edited on 2-19-2016 by nlegaux]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by nlegaux  |
Also, would it be more appropriate to move this thread to organic chemistry or technochemistry until I have created a finalized procedure?
|
I think that is a good idea until you can verify your target product and quantify a yield.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by nlegaux  |
I did a bit of work with the cell yesterday, and in the process I corroded one of my electrodes to a point that it is no longer useful. I think I will
make a few more lead electrodes for experimentation in the immediate future, but I will definitely need to investigate more corrosion-resistant
electrode materials (perhaps platinated titanium?).
Also, would it be more appropriate to move this thread to organic chemistry or technochemistry until I have created a finalized procedure?
|
You're probably anode oxidising lead to lead sulphate. Not a big problem but better avoided nonetheless.
PtTi sounds expensive and may not even be that effective, unless the coating is very good. Ti is corroded easily to purple/blue
Ti<sup>3+</sup> (a strong reducing agent) by warm dilute H2SO4.
Why not consider good old graphite, for anode?
Re. your last point, that's a moderator's responsibility (contact Bert or zts16 or Nicodem) but I'd favour splicing this thread onto the first link in
your post. It would revive an old but interesting thread, until your method's teething problems have been sorted out.
[Edited on 19-2-2016 by blogfast25]
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Mn(OH)2 to MnO2 by air oxidation sounds interesting!
Could you please site a reference or an experimental procedure showing how to do it?
gsd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gsd  |
Mn(OH)2 to MnO2 by air oxidation sounds interesting!
Could you please site a reference or an experimental procedure showing how to do it?
gsd |
Simply dry the Mn(OH)2 slurry (moist filter cake), preferably with some alkali in it, on a hot plate: it oxidises to MnO2 almost
as quickly as Fe(OH)2 to Fe2O3.
Keep stirring and turning until it's all brown/black, wash out the (optional) alkali and dry and semi-calcine.
Of course it's almost instantaneous if you use bleach (hypochlorite).
<hr>
And then there's this, from a google book:

It's the reference to:
MnO2 + Mn<sup>2+</sup> + 4 H<sup>+</sup> <===> 2 Mn<sup>3+</sup> + 2 H2O
... claimed to be fast from left to right that I find interesting.
[Edited on 20-2-2016 by blogfast25]
|
|
|
Texium
|
Thread Moved
19-2-2016 at 17:17 |
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I moved the thread to Organic Chemistry since it was requested by the OP.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
For the characterization of acetaldehyde...
-acetaldehyde imine trimer (2,4,6-trimethyl-1,3,5-perhydrotriazine) should be cristaline
-acetaldehyde cyclic trimer (paraldehyde)
-acetaldehyde cyclic tetramer (metaldehyde)
And here pertinent pages 44,45 and 67 from
"Spectral & Chemical Characterization of Organic Compounds - by W.J.Criddle and G.P.Ellis - A Laboratory Handbook - 3rd edition - WILEY"
Making of the following derivatives
1°) 2,4-dinitrophenylhydrazone
2°) Semicarbazone
3°) Oxime
4°) 4-nitrophenylhydrazone and phenylhydrazone
5°) Dimethone

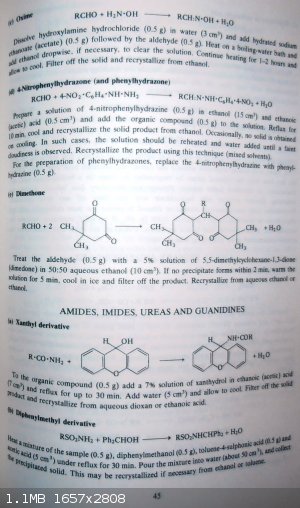
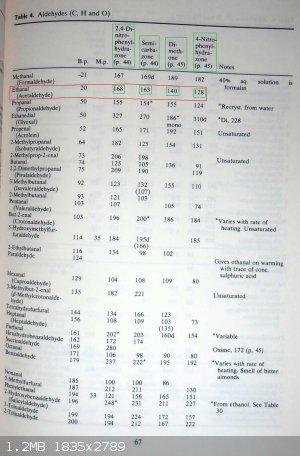
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
My graphite electrodes finally came in the mail. They seem to be working quite well; after running for a few hours I see little corrosion. On this run
of the cell rather than maintaining the cell at 70*C using heating, I have placed it into a bowl of water and it maintains its own temperature at
around 50*C.
However, I have found an issue. I have not been able to collect any distillate on this run of the cell. As a test, I removed a small amount of
electrolyte and added it to a test tube. It is a brown/black clear color (see photos below) with a small immiscible layer on top. I than took about
1ml of the collected electrolyte and added about 1ml of Fehling's solution. After heating, the test was negative (which is to be expected because of
the lack of distillate). Next, I added a small amount of ethanol and repeated the test. It indicated again that no aldehydes were present.

The collected electrolyte. My apologies about the poor photo quality, I couldn't get my phone camera to focus on the test tube. The immiscible layer
is too small to be clearly visible in this photo.
Because of this information, I believe I may know what is going on. I think the ethanol is being over-oxidized to ethanoic acid which is forming an
ester in the highly acidic environment with the unreacted ethanol forming ethyl acetate (hence the immiscible layer). This ethyl acetate would not
boil off at the apparatus's operating temperature of 50*C, causing it to remain in the reactor and possibly consume the oxidant (that last part is
purely a guess. I have not been able to find any references to oxidizing ethyl acetate to carbon dioxide and water, but it certainly seems plausible.
Also, this would fall in line with the test indicating that the electrolyte was unable to oxidize the ethanol). I will attempt to distill this
immiscible liquid off in order to better characterize it. In previous runs of the cell, I was probably distilling mostly ethyl acetate with a small
amount of acetaldehyde dissolved into it, which would fall in line with my observations on the original product work up (a small amount of material
boiling at 23.4*C with the majority of the liquid left in the boiling flask). In the mean time, are there any ideas on how to remedy this issue,
assuming it is ethyl acetate formation?
nlegaux
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@nlegaux:
Ouch.
I'm now beginning to think the method may only be suitable for certain aldehydes, like benzaldehyde. The latter is of course poorly soluble
in water, while ethanal is totally miscible with it. That makes the further oxidation of the latter to ethanoic acid much easier. The
Mn(+3)/Mn(2+) system is a very strong oxidiser.
The formation of the ester is of course plausible, considering the high concentration of H2SO4 in the mix.
The formation of EtOAc may even promote the further oxidation of ethanal to ethanoic acid by Le Chatelier.
[Edited on 25-2-2016 by blogfast25]
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Could this problem possibly be fixed by introducing an immiscible organic phase to dissolve the ethanol/ethanal?
The distillate is coming over at approximately 70*C (lit for ethyl acetate is 77.1*C, but the barometric pressure here today is about 750mmHg). It is
a slight yellow color and immiscible with water. Its odor is the same as that of a known sample of ethyl acetate I have on hand. I found the density
to be 0.954g/ml, which is similar to the reported value for a ternary azeotrope of ethyl acetate, ethanol, and water (lit. 0.901g/ml).
Worst case scenario I may be able to make an ethyl acetate producing cell 
nlegaux
[Edited on 2-25-2016 by nlegaux]
|
|
|
| Pages:
1
2 |