DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
A less reviewed explosive with VoD 10km/s
I see little information on DNAF on this forum 4,4'-Dinitro-3,3'-diazenofuroxan, AKA
(4,4'-DiNitro-3,3'-diAzenoFuroxan)
From past forum posts, i see that there has been little confusion few members here thought this DNAF was synthesized by a notable member in the past
Axt, however, the DNAF Axt synthesized was 3,3'-Dinitroazoxyfurazan, and hence the name
3,3'-DiNitroAzoxyFurazan
Link to Axt's DNAF(C4N8O7) synthesis: http://www.sciencemadness.org/talk/viewthread.php?tid=5813
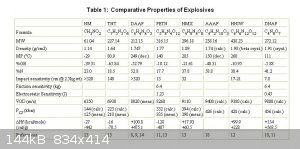
The DNAF Axt synthesized has a high density of over 1.9 and a VoD of 9800, however The DNAF (C4N8O8) this post is about has a claimed density of 2.02
and a detonation velocity of 10km/s.
A short summery of DNAF and its synthesis can be found here on the defense science journal.
Direct download link: http://www.publications.drdo.gov.in/ojs/index.php/dsj/articl...
an abstract of the original paper reporting the synthesis of DNAF is here, however I am not sure how to get it, if anyone can find it that'd be great.
http://www.maik.ru/contents/danchem/danchem4-6_98v359cont.ht...
[Edited on 3-1-2016 by DubaiAmateurRocketry]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
It is my first time to know about this EM. Thx a lot.
will this link help you : Link
I am thinking to prepare it but I think it is a bit sensitive.
Did you try it before?
[Edited on 3-1-2016 by ecos]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Did find these though
a patent ( I believe I've seen this posted somewhere )
https://translate.google.com/translate?hl=en&sl=zh-CN&am...
Synthesis of 3-Amino-4-nitrofurazan by an Improved Method , is a chinese paper
www.energetic-materials.org.cn/hnclen/ch/reader/create_pdf
Synthesis of Energetic Furazan Derivatives
https://dspace.iup.edu/bitstream/handle/2069/761/Bryan%20J.%...
Calculation of Thermochemical and Explosive Characteristics of Furoxanes
is also here
http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.bazt...
or here
www.wydawnictwa.ipo.waw.pl/cejem/Number-3-4-2008/Zhukov.pdf
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by ecos  | It is my first time to know about this EM. Thx a lot.
will this link help you : Link
I am thinking to prepare it but I think it is a bit sensitive.
Did you try it before?
[Edited on 3-1-2016 by ecos] |
I have not yet tried synthesizing any of the compounds. Furazans is a very attractive class of energetic materials that I wish I had a hand on to play
with, however, college is very busy and distracting hehe.
If you ever try synthesizing sensitive compounds such as DNAF, be sure to take a lot of precaution and in very low quantity.
[Edited on 3-1-2016 by DubaiAmateurRocketry]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Unfortunately , I couldn't find any nitromethane in stores in my country 
I will not be able to synthesis anything  :'( :'(
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by ecos  | Unfortunately , I couldn't find any nitromethane in stores in my country 
I will not be able to synthesis anything  :'( :'( |
nitromethane should be easy to synthesize and purchase, furthermore, I don't think you have to have nitromethane to synthesize it. If i remember
correctly, furazans has more than one method of synthesis and you can possibily start from glyoxime if you can find it.
Thanks a lot for these Franklyn, however I cannot open the Chinese paper you posted.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
www.energetic-materials.org.cn/hnclen/ch/reader/create_pdf.a...
www.google.com/#q=Synthesis+of+3-Amino-4-nitrofurazan+by+an+...
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Question: DubaiAmateurRocketry was an account shared by several different people in the past. This led to some confusion at times.
Is this still the case, or can we expect that posts under this screen name originate from a single author now and going forward?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Thank you franklyn.
And yes you can assume posts from this account originate from a single author now on. It is still technically shared however the others no longer use
this forum.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
There is a wikipedia link for it but its accronym is DDF and not DNAF
4,4'-Dinitro-3,3'-diazenofuroxan
It is a perfect OB, aromatic, hydrogen-less molecule...as such it is indeed very powerfull...
[Edited on 5-1-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
The lack of popularity comes from the great difficulty involved in the manufacture of furoxans in an amateur setting.
Those who are genuinely interested in the chemistry behind these complex molecules soon find there are much safer ways to tinker with advanced areas
of chemistry without constantly dealing with intermediates that could kill you at any moment. For all others, there are simpler ways to make
powerfully destructive materials with much less time, effort, and expense. Thus, those with the particular tenacity required reach the cutting edge of
amateur EM are rare, and as such amateur work on furoxans is very limited.
Kudos are deserved to those who do make these amateur breakthroughs in exotic EMs, but take it from someone in the industry that the experimental EM
world is full of pulverized glass and scarred hoods, and is readily disfiguring to those underprepared for the inevitable.
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
DAR,
The synthesis of 4,4'-dinitro-3,3'-azofuroxan or 4,4'-Dinitro-3,3'-diazenofuroxan (DNAF) is a lengthy multi-step procedure. I've detailed the
synthesis of DNAF in the following scheme:
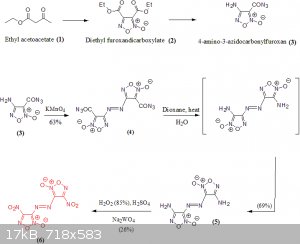
The most important precursor for the synthesis of DNAF is 4-amino-3-azidocarbonylfuroxan (3), synthesized from Diethyl furoxandicarboxylate (2) which
in turn can be obtained from Ethyl acetoacetate (1). Procedure on how (2) can be obtained from (1) can be found in reference [1].
Compound (3) can undergo an oxidative coupling in the presence of KMnO4 to generate (4). A Curtius rearrangement is obtained when (4) is
heated in Dioxane in the presence of water. This rearrangement led the formation of the corresponding diamino derivative (5) in 69% yield. The final
step is the oxidation of the diamino to the corresponding dinitro and this by using harsh condition [2]: 85% concentration of hydrogen peroxide in 96%
sulfuric acid all this in the presence of sodium tungstate. The oxidation step as seen in the scheme above is inefficient due to the low yield of this
reaction (26%).
As claimed in [2], the detonation velocity and pressure of DNAF (at 1.97 g/cm3) are D= 9.70 km/s, P= 470 kbar. The single crystal density
of DNAF is 2.002 g/cm3 (obtained from X-ray diffraction data). At this density the detonation velocity will reach 10 km/s.
DNAF and other furazan/furoxan derivatives were investigated as ingredient in composite propellant formulation, for more information see the attached
file below.
References:
[1] Snyder, H. R., & Boyer, N. E. (1955). The Synthesis of Furoxans from Aryl Methyl Ketones and Nitric Acid1. Journal of the
American Chemical Society, 77(16), 4233-4238. doi: 10.1021/ja01621a021
[2] Makhova, N. N., & Kulikov, A. S. (2013). Advances in the chemistry of monocyclic amino- and nitrofuroxans. Russian
Chemical Reviews, 82(11), 1007.
Dany.
Attachment: Propellants formulations.pdf (284kB)
This file has been downloaded 679 times
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by DubaiAmateurRocketry  | Quote: Originally posted by ecos  | Unfortunately , I couldn't find any nitromethane in stores in my country 
I will not be able to synthesis anything  :'( :'( |
nitromethane should be easy to synthesize and purchase, furthermore, I don't think you have to have nitromethane to synthesize it. If i remember
correctly, furazans has more than one method of synthesis and you can possibily start from glyoxime if you can find it. |
I would need NM to synthesis Hydroxylamine Hydrochloride!
do you know simple route to synthesis NM ? I think i need to nitrate propane and then filter. This is not a simple task.
NM is not sold in all countries. I also don't know where to get glyoxime !
so sad 
[Edited on 12-1-2016 by ecos]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by DubaiAmateurRocketry  | Quote: Originally posted by ecos  | Unfortunately , I couldn't find any nitromethane in stores in my country 
I will not be able to synthesis anything  :'( :'( |
nitromethane should be easy to synthesize and purchase, furthermore, I don't think you have to have nitromethane to synthesize it. If i remember
correctly, furazans has more than one method of synthesis and you can possibily start from glyoxime if you can find it. |
I would need NM to synthesis Hydroxylamine Hydrochloride!
do you know simple route to synthesis NM ? I think i need to nitrate propane and then filter. This is not a simple task.
NM is not sold in all countries. I also don't know where to get glyoxime !
so sad 
[Edited on 12-1-2016 by ecos] |
https://en.wikipedia.org/wiki/Nitromethane#Laboratory_method...
This is a fairly simple method.
Both starting materials are not exotic and it is a fairly straightforward single step synthesis. Make sure to find out how to clean any side products
such as salt out of nitromethane if they are soluble. I have not yet checked and ill look into it later when I get back.
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
You could also synthesize HO-NH3*HCl directly (NaNO2, sulfite, SO2, hydrochloric acid and acetone) (see OrgSyn: http://www.orgsyn.org/demo.aspx?prep=CV1P0318).
The main problem here would be the practicalities of SO2 production (at least for me, but that is mostly because I would like to start from elemental
sulfur). A good small-scale solution might be to generate it from HCl and sulfite.
[Edited on 13-1-2016 by Microtek]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I have performed the orgsyn procedure on a smaller scale with good results. SO2 is easy to generate; just add acid to a sulfite, bisulfite, or
metabisulfite salt. The main drawback is that this prep requires bucket-sized labware for small amounts of hydroxylamine, something like 20g per liter
of reaction space.
I also tried modifying the procedure to use metabisulfite rather than bisulfite since metabisulfite acts like bisulfite + SO2 in solution, and I
thought maybe the SO2 addition step could be skipped this way. However, there was no product at the end, so I resulted in bubbling SO2 for a few
hours.
The procedure is a pain in the ass, but unless you can get your hands on chloroacetic acid, it's the next best thing.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nitromethane has been discussed many times into the forum.
I would go for known processes:
CH3-I or CH3-Br in DMF with AgNO2 or LiNO2/urea (NaNO2) in aceton.
Heating over 200°C and below 400°C of exces butane, propane or even better ethane bubbled into 69% HNO3 with condensation of nitromethane.
Or new unexplored ones:
Maybe heating of an exces acetic acid or acetonitrile with concentrated HNO3...the resulting nitrated compounds should decarboxylate.
Maybe heating of 1,1,1-trichlorethane with HNO3 and basic hydrolysis.
Maybe nitration of malonic acid or ester...and di-decarboxylation.
For Hydroxylamine there is also reduction of NaNO2 with Zn and aceton see into the reference section requested documents 9 by PHILOU Zrealone
The document is given by solo a little above my post into the link...
[Edited on 13-1-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|