Copper
Harmless

Posts: 17
Registered: 24-10-2015
Member Is Offline
Mood: No Mood
|
|
Purple floating liquid on CuCO3?
Hello all,
After synthesis and filtration of CuCO3 I heated it on a hot plate. The solid melted ??? and formed a strange purple floating layer on the top ???
Does anyone know the cause of that?
Thanks
 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You need to describe your synthesis first. W/o it no one can make any significant pronouncements re. what is causing the purple colour.
|
|
|
Copper
Harmless

Posts: 17
Registered: 24-10-2015
Member Is Offline
Mood: No Mood
|
|
It's the standard NaHCO3 and CuSO4 precipitation.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You need to specify quantities.
I think poorly washed basic copper carbonate (CuCO3 DOESN'T exist, only basic copper carbonate does) gave cuprate which is blue. Blue +
green gives purple.
|
|
|
Copper
Harmless

Posts: 17
Registered: 24-10-2015
Member Is Offline
Mood: No Mood
|
|
Ok thanks,
As I had a good amount of very concentrated (near saturated) copper sulfate solution I just added baking soda until effervescence ceased. It was
filtered using coffee filters and washed twice with distilled water. Then it was scooped and placed into the 100mL beaker.
Also I don't think blue and green will give purple, instead it gives turquoise.
|
|
|
Texium
|
Thread Moved
29-12-2015 at 20:43 |
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
Remarkable.
- - - - - - - - - - - - - - - - - - - - - - - - - -
Dubious.

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No.
Many know I have 'a thing' for basic copper carbonate, primarily because of the stubborn belief in the non-existent CuCO3.
See also this lengthy thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=50822
Methinks you have a bit of a 'grudge management problem' that might be clouding your judgement somewhat.
[Edited on 30-12-2015 by blogfast25]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
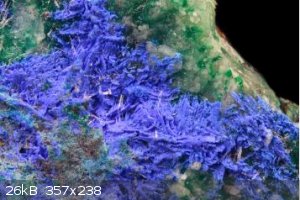
There are several normal double carbonates of copper and other elements; check out Juangodoyite it ranges from deep blue violet to a lilac colour. It
is Na2Cu(CO3)2 but sparingly soluble but other ratios of Na to Cu may be more soluble, looks reasonable.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | There are several normal double carbonates of copper and other elements; check out Juangodoyite it ranges from deep blue violet to a lilac colour. It
is Na2Cu(CO3)2 but sparingly soluble but other ratios of Na to Cu may be more soluble, looks reasonable.
|
Nice find and I'd never heard of that mineral before. But I find it hard to see how it could form in OP's conditions, though...
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Quote: Originally posted by Boffis  | There are several normal double carbonates of copper and other elements; check out Juangodoyite it ranges from deep blue violet to a lilac colour. It
is Na2Cu(CO3)2 but sparingly soluble but other ratios of Na to Cu may be more soluble, looks reasonable.
|
Are there any methods of making these double carbonates?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Maybe heating mixture of Cu2(OH)2CO3+ Na2CO3+ NaHCO3 up to boiling point of water but under calcination of NaHCO3 it is just a guess but not so hard
to try so good luck.
|
|
|