Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Triphenylphosphine Synthesis?
While doing reading on Carbon Tetrabromide,I came across a compund called Triphenylphosphine. After reading about Triphenylphosphine, I came across an
article that states that it could be used the Mitsunobu Reaction,the Appel Reaction, deoxygenation, and sulfonation. I am more interested in the first
2 reactions,so my question is: How can I synthesize triphenylphosphine?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
You can buy 100 g of it on eBay for $28 including shipping.
[Edited on 24-10-2015 by careysub]
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
100 grams for 428$ is affordable yet does not appear to be a cost-effective trade-off. I created this thread asking for synthesis methods,please don't
tell me to buy it(Unless it's the only method of obtaining it).
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
You can synthesize triphenylphosphine by the reaction of phosphorus trichloride with phenylmagnesium bromide. I believe there is a procedure
somewhere on this forum.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by gdflp  | | You can synthesize triphenylphosphine by the reaction of phosphorus trichloride with phenylmagnesium bromide. I believe there is a procedure
somewhere on this forum. |
Can you please provide a link?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Agari  |
100 grams for 428$ is affordable yet does not appear to be a cost-effective trade-off. I created this thread asking for synthesis methods,please don't
tell me to buy it(Unless it's the only method of obtaining it). |
The wise words of Dan Vizine (I think) turn this around, don't make something unless you can't buy it (due to cost, lack of sources, of not wanting to
attract attention) - unless it is really the synthetic exercise you are after. If you just want it to employ in some other reactions, buying it is the
best idea.
Nobody cares if you buy triphenylphosphine (to the best of my knowledge) - it is cheap and readily available.
But it appears extremely costly and difficult to make in the home lab, requiring reagents like phosphorous trichloride AND phenylmagnesium AND sodium
metal. Sodium azide is also used in other methods.
A lot of chemicals are like this, easy and cheap in industrial scale processes all but impossible at home.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by careysub  | Quote: Originally posted by Agari  |
100 grams for 428$ is affordable yet does not appear to be a cost-effective trade-off. I created this thread asking for synthesis methods,please don't
tell me to buy it(Unless it's the only method of obtaining it). |
The wise words of Dan Vizine (I think) turn this around, don't make something unless you can't buy it (due to cost, lack of sources, of not wanting to
attract attention) - unless it is really the synthetic exercise you are after. If you just want it to employ in some other reactions, buying it is the
best idea.
Nobody cares if you buy triphenylphosphine (to the best of my knowledge) - it is cheap and readily available.
But it appears extremely costly and difficult to make in the home lab, requiring reagents like phosphorous trichloride AND phenylmagnesium AND sodium
metal. Sodium azide is also used in other methods.
A lot of chemicals are like this, easy and cheap in industrial scale processes all but impossible at home. |
Oh lol,I thought that your price tag read 428$. I would like to synthesize it as a challenge,but if it can't be done,I may just simply buy it.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
If you want to do it for funsies, buy the PPh3 and make a small amount of SbPh3. Antimony halides are much easier to make and handle (and obtain
starting materials for) than phosphorus halides. The product, unfortunately doesn't do Wittigs and similar reactions but can be quaternized and turned
into a ylide.
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
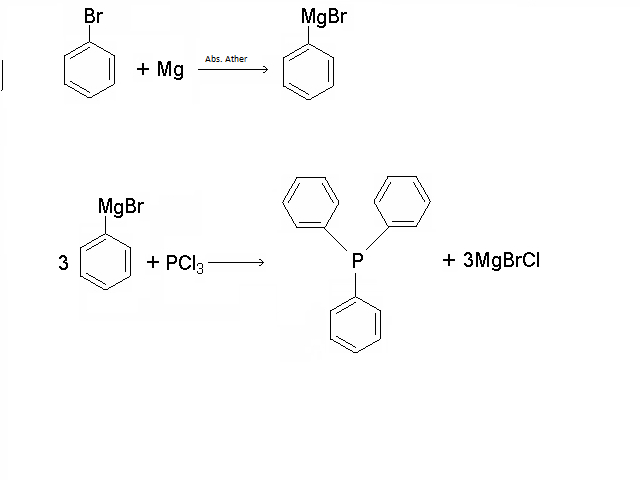
Grignard process (easy), you can translate with google: http://forum.lambdasyn.org/index.php/topic,2082.0.html
Indrustial pathway with molten sodium (need good practice):
http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=CA1123460&...
[Edited on 25-10-2015 by Aqua-regia]
[Edited on 25-10-2015 by Aqua-regia]
|
|
|
krishna
Harmless

Posts: 1
Registered: 25-11-2015
Member Is Offline
Mood: No Mood
|
|
Melt Sodium metal in xylene under vigorous stirring and add phosphorus trichloride. It forms
|
|
|