karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Condensation of Mandelic acid ester with Guanidine
Hello there,
I have a problem which is really frustrating as I don´t know why it keeps happening.
I am trying to condense guanidine freebase with ethyl mandelate, to prepare an oxazolidinone.
The mandelic acid ester was prepared via standard fischer esterification, using 1-1,5ml H2SO4 per 0,1mol of mandelic acid and about 150ml dry ethanol
per 0,1mol mandelic acid.
That worked well.
Also the preparation of guanidine freebase from its hydrochloride and ethanolic sodium hydroxide. In the very first I used sodium metal, dissolved in
ethanol, and did not filter of the precipitated NaCl. Also not the next time.
The last few times I filtered off the NaCl and evaporated off most of the ethanol before adding the mandelate.
But my problem that keeps happening is, after I added the mandelate ester, and the reaction gives off ammonia like it should be (I tried the reaction
in the cold, letting it stand over night, at reflux temperature for a shorter period), I can´t precipitate the product from the reaction mixture.
After the reaction is over, means it does not give off anymore ammonia, I added water and nothing happened. I added HCl until a pH of 2-5 is reached
(did it several times, so different values), and also nothing happened.
Because the reaction proceeds with release of ammonia, it should have reacted.
Even if the mandelate ester is added while the ethanolic guanidine solution is cold, after a few minutes ammonia is released.
So why can´t I get the product out of it? It should be insoluble in acidic media, but soluble in alkaline ones.
The references I used are mostly very vague on the precipitation, so I guess it should very easily precipitate. But it doesn´t for me.
I would try a solvent extraction, if I would just knew which one to use for that.
In my experiments, I used 2eq. guanidine to 1eq. ethyl mandelate, also two experiments with only a 1:1 ratio.
At the moment I have one running which will be worked up like in example 2 in the first reference.
Meaning, I will try to dissolve the residue after I distilled off the ethanol, in NaOH solution, filter it, the treat the filtrate with AcOH to
precipitate whatever I produced if anything.
Can somebody give helpful advice?
The references I used are:
http://www.google.com/patents/US3029189
http://chemistry.mdma.ch/hiveboard/methods/000511479.html#
https://www.erowid.org/archive/rhodium/chemistry/pemoline.ht...
https://www.thevespiary.org/talk/index.php?topic=12264.5;wap...
Attachment: phenyl isohydantoin, in german (389kB)
This file has been downloaded 446 times
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Try saturating the solution with NaCl after adding water and acidifying. Extract with Et2O and see if you get anything.
If not, I'd suspect the starting materials. Did you take a MP of your ethyl mandelate?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yes, it refused to crystallise directly after extraction, but after distillation in vacuo, it is melting around 32-33°C.
I don´t know about its solubility in ethers, additional I know that 1% is soluble in propylen glycol.
One reference about its solubility says, "l: Propylenglykol 1%, heißer EtOH, pul: H2O, Eth, verd. HCl"; while I guess means the "l" stands for the
translation of soluble(=löslich), I am not sure yet what "pul" means. These informations are from "Hagers Handbuch der pharmazeutischen Chemie".
I guess it´s something for insoluble, that would probably mean with "Eth", ethers are meant.
But I will try to extract the reaction medium directly after the reaction, but with MTBE instead of Et2O. Also addition of NaCl.
Another thing, as ammonia evolves, it should have reacted.
I have drawn the reaction here:
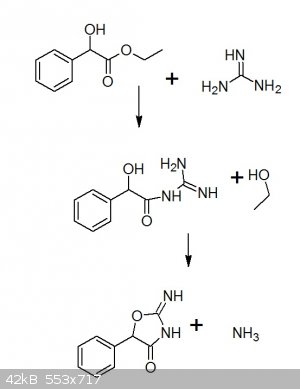
One can see that in the first step(like it is said in the added pdf in my starting post), it is an ammonolysis of an ester, which first gives the
guanidine derivate and EtOH, which in the second step cyclises under release of NH3.
I guess it´s my work-up why it seems to fail everytime. As obviously the reaction proceeded like it should.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I'm getting a lot of contradictory references on solubility as well. From the .pdf reference you attached comes this:

Which says, "The crystals of the compound dissolve fairly easily in hot alcohol, more readily in hot water, and on cooling of the solvent almost
completely back out."
But I'm also assuming that if it's not soluble in cold water/alcohol ("almost completely back out"), it will probably be soluble in Et2O at that
point. MTBE should work just as well. Let me know how it works out.
I also wonder if maybe it doesn't work with whichever enantiomer of mandelic acid you used. Was it enantiopure?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Pemoline is practically insoluble in water, alcohols, ethers and just about any nonpolar solvent. It cannot be extracted with any solvent, simply
because it is insoluble. It crystallizes immediately. If you don't get any precipitate after you neutralize the reaction mixture, then your reaction
did not work. Smelling ammonia is not evidence that the reaction occurred.
I suggest you to use methanolic NaOMe to form a guanidine methanolic solution. Do not concentrate it and do not heat it before you add ethyl
mandelate. Make sure to avoid any presence of water, so do not use NaOH.
The chirality of the mandelate is irrelevant.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I´ve used racemic mandelic acid.
Well, I will try to extract the product with MTB-ether and report back.
The solubility data is irritating, I can´t find a reputable source.
Edit: Thank you Nicodem for your information, I will try it again using sodium ethoxide. I haven´t seen your post before replying.
I will use a ethanolic sodium ethoxide solution because scrambling of the ester would be a problem as I only have the ethyl ester. I hope it will work
equally well.
[Edited on 25-11-2015 by karlos³]
[Edited on 25-11-2015 by karlos³]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  | Edit: Thank you Nicodem for your information, I will try it again using sodium ethoxide. I haven´t seen your post before replying.
I will use a ethanolic sodium ethoxide solution because scrambling of the ester would be a problem as I only have the ethyl ester. I hope it will work
equally well. |
If you can, use NaOMe and methanol instead. Ester scrambling is irrelevant for the reaction and the transesterification to the methyl ester under the
reaction conditions will be very fast. The scrambling might confuse a bit the TLC monitoring at the beginning, but pemoline will not go nearly as far
up on silica anyway, it will probably stay immobile on the start. The methyl ester is more reactive and the reaction should be more efficient in
methanol regardless of the lower reflux temperature.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Ok thank you for that piece of information, also about the behaviour of the substances on TLC.
It is very good that you said that, as I have a bit of MeOH at the moment, but not as much as needed for making the methyl ester.
Nicodem, as always you have been very helpful! 
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The ester has nearly disappeared on TLC!
I´ve used now my last bit of MeOH, the sodium dissolved slowly, then added the guanidine-HCl in MeOH after it was cooled, NaCl precipitated as
expected, and then added the mandelate while still cold. It instantly gave off ammonia and then I´ve refluxed till two hours ago.
I will let it stir till tomorrow and then work it up.
Thank you for your advice Nicodem, it seems to work much better now!
Edit: It has worked. Thank you Nicodem, you are really one of the good guys 
The product is still drying in the filter.
I am trying to get more MeOH now. This was the first try using MeOH and also the first that worked. Next time I will directly make the methyl ester.
[Edited on 27-11-2015 by karlos³]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
So, for completion I would like to write what I got:
1,44g (0,062mol) Na were dissolved in 25ml MeOH and after it dissolved (took much longer than dissolving Na in EtOH) the solution was cooled and 6g
(0,062mol) of guanidine HCl in 20ml MeOH were added.
Instantly NaCl precipitated.
Next, 11,3g of ethyl mandelate were added in 10ml MeOH, and ten minutes later I started to reflux, for five hours until TLC indicated that the ester
has reacted. The reaction mixture was allowed to stand until the next day, until 100ml dH2O and enough 7% HCl were added (around 50ml). The NaCl
dissolved and the solution got a little bit warm, but instead of complete dissolution of everything that made the reaction mixture murky, a lot of
white particle "precipitated" (they did not really precipitate, as it seemed, it looked more like they or some of them were already there).
The precipitate was filtered, washed with dH2O three times (50ml each), then two times with 30ml EtOH.
Then they were air dried to provide a yield of 8,1g, corresponding to 73% of the theory.
Thanks for the advice! 
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
Hi,
I think you maybe killed the guanidine with NaOMe by transforming it into some kind of carbamate with the release of ammonia before adding the
mandelate. Ammonia is a gas and will be spread off and thus balance the reaction to the right. Did you smell ammonia during the neutralisation with
NaOMe ? Do you have still some base in the MeOH after neutralisation ? NaCl is not a problem here it's just annoying.
|
|
|