| Pages:
1
2
3 |
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
A thought entered my mind today.During the decomposition of the HMTA-adduct,is there a chance of a substitution reaction between the benzylic OH and
Cl- from the HCl to form chlorophenyl ethylamine ?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Well, certainly not in an aqueous environment, if you want to chlorinate such an alcohol, you would need pinch of a catalyst like ZnCl2, otherwise the
chloro-compound would instantly hydrolyse back to the aminoalcohol, with chiral inversion.
In fact, this is a known route to racemise an alcohol in similiar compounds like (nor)ephedrine.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  | Well, certainly not in an aqueous environment, if you want to chlorinate such an alcohol, you would need pinch of a catalyst like ZnCl2, otherwise the
chloro-compound would instantly hydrolyse back to the aminoalcohol, with chiral inversion.
In fact, this is a known route to racemise an alcohol in similiar compounds like (nor)ephedrine. |
I had this video in mind when I said that
https://www.youtube.com/watch?v=mtl-Hs66Uzo
they don't use any ZnCl2 though
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Do you have access to tosyl chloride by any chance? If so, what about doing a Neber rearrangement on acetophenone to get 2-amino-1-phenylethanone? You
could then reduce the aminoketone to the desired aminoalcohol with something like sodium borohydride. The Neber rearrangement should give pretty good
yields (70-75%), and the reduction should give extremely good yields. The only thing to watch out for is dimerization since it's an alpha-aminoketone
(and a primary one at that). Just protect the carbonyl group by initially reacting the intermediate azirine with acidic alcohol to form an acetal.
Then when you're ready to reduce it, just hydrolyze it to the ketone. I'd also try to keep it in its salt form, too.
Anyway, just something to consider if you're not having much luck with any of the other synthesis routes you've tried.
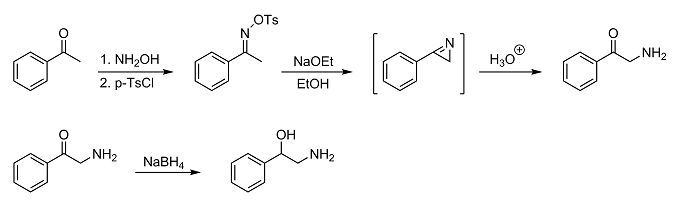
Neber rearrangement from OrgSyn using 4-Acetylpyridine instead of acetophenone
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Delépine reaction on acetophenone? Phenacyl bromide should be an optimal substrate for the reaction... but it is a tear gas so do be careful if you
go that route. The ketone can be reduced with any number of chemicals; Al(OiPr)3 is an obvious choice, but Na2S2O4 or NaBH4 should work fine.
I'm surprised the Pd/C didn't work out for you. Maybe give it another go with a different solvent? The papers use MeOH/THF but you could try
MeOH/EtOAc or just EtOAc.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Once again I don't understand the goal of the discussion. There's no way to make 2-amino-phenylethanol easier than by reaction of epoxide with
amine/imine/imide. Unless you prove me wrong, but I see no arguments.
4 steps route is cool, but it's a fucking pain in the ass with tosyl chloride, sodium ethoxide and sodium borohydride.
I propose to react glyoxalic acid with benzene, then separate mandelic acid, convert to amide, and finally reduce the amide with LAH.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Those contributions are very worthful, thank you all very much!
@CuReUS: Thats because benzyl alcohol is a primary one, those get easier halogenated without a catalyst than secondary alcohols like phenethanolamine
is one.
@Darkstar: Well thats an interesting route, but I would probably more likely reduce isonitrosoacetophenone for that matter, I know dimerisation would
be a big problem even then, don´t want any dihydropyrazine dimer into it. That´s why I stay away for the moment from acetophenone as a precursor.
But if I wanted any specific isomer, then it would be the educt/starting material of my choice.
@clearly_not_atara: I am wondering too about my Pd/C, I am planning another try with another substrate and another solvent system, but I am not sure
if it maybe is of lesser quality than advertised. I bought it cheap from someone I know, not in a shop, not originally packed, so who knows? Another
experiment will show.
Delepine on Phenacyl halide sounds also good, I know its a bad lachrymatory, as if have already encountered bromopropiophenone 
It would be interesting to know if this reaction also have to be made in CHCl3 like for styrene oxide/styrene halohydrin and if the hexamine adduct
also contains "crystal-chloroform"?
I am unsure if the delepine on styrene oxide would also work in DCM, as it is much cheaper than CHCl3? That would be a lot more supportive for this
experiment.
I have tried the reaction already in EtOH but like every paper confirms it does not work well if anything. What also goes along with my experience.
Now I know that the chloroform is essential for this reaction, but it would be much better if it can be substituted with another solvent.
Of course I will try it with chloroform first, it is maybe a bit more expensive, but I will follow the literature exactly for my next attempt.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by byko3y  | | Once again I don't understand the goal of the discussion. There's no way to make 2-amino-phenylethanol easier than by reaction of epoxide with
amine/imine/imide. Unless you prove me wrong, but I see no arguments. |
I think the problems with the epoxide
route are the lability of styrene epoxide, the inaccessibility of styrene monomer (depolymerization is a pita), and the difficulty in obtaining
reagents used to form the epoxide, preferably mCPBA, which isn't too hard to make (it's on orgsyn) but it's not the sort of thing you can buy at the
store.
Actually I've always wondered if you can make mCPBA from benzoic acid. Orgsyn uses the acyl chloride, but there is a difference in solubility in DCM
between the acid and peracid that might allow you to shift the equilibrium in the presence of H2O2.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Looks to me that synthesis via hydrogenation of Benzaldehyde Cyanohydrin is promising.
Working with cyanide isn't fun, but it looks like Benzaldehyde Cyanohydrin, may be commercially available.
https://books.google.com/books?id=nacWAAAAQBAJ&pg=PA613&...
There is also some prep information available via erowid: https://www.erowid.org/archive/rhodium/chemistry/mandelic.ht...
[Edited on 14-10-2015 by zed]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
@zed -
I posted a good reference for the prep you speak of. See above, 5th reply in this thread.
It is confusing because of nomenclature. Benzoyl cyanide = benzaldehyde cyanohydrin.
LiAlH4 or hydrogenation can be used, but the hydrogenation workup is going to suck.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  |
I propose to react glyoxalic acid with benzene, then separate mandelic acid, convert to amide, and finally reduce the amide with LAH.
|
how will you make mandelic acid from glyoxalic acid and benzene? don't tell me you have a FC-acylation followed by an intramolecular cannizaro in mind

Quote: Originally posted by karlos³  |
@CuReUS: Thats because benzyl alcohol is a primary one, those get easier halogenated without a catalyst than secondary alcohols like phenethanolamine
is one. |
But I thought that secondary alcohols undergo faster substitution without catalyst than primary alcohols.Also the -I effect of the NH2
group should speed up the reaction,shouldn't it ?
Quote: Originally posted by karlos³  | That´s why I stay away for the moment from acetophenone as a precursor. But if I wanted any specific isomer, then it would be the educt/starting
material of my choice.
|
Karlos,how do you plan to do the enantioselective synthesis ? will you use a chiral reagent ?
| Quote: | | Now I know that the chloroform is essential for this reaction, but it would be much better if it can be substituted with another solvent.
|
I suggested using DMF in the previous page.
styrene is available OTC as fibreglass resin
| Quote: | | the difficulty in obtaining reagents used to form the epoxide, preferably mCPBA, which isn't too hard to make (it's on orgsyn) but it's not the sort
of thing you can buy at the store. |
yes you are right.But karlos is not going to use mCPBA in the first place.There are many alternate,amateur friendly and OTC reagents to make the
epoxide.
| Quote: | | Actually I've always wondered if you can make mCPBA from benzoic acid. Orgsyn uses the acyl chloride, but there is a difference in solubility in DCM
between the acid and peracid that might allow you to shift the equilibrium in the presence of H2O2. |
I don't see why not.It is well known that performic and peracetic acid can be made from the acids itself.And benzoic acid is stronger than acetic
acid.IIRC perbenzoic acid can be made from benzoic acid.But you need methane sulphonic acid and 70% H2O2 
I already suggested this "prep" in my post just below the one you have linked.Read the last line
| Quote: | | It is confusing because of nomenclature. Benzoyl cyanide = benzaldehyde cyanohydrin. |
I am sorry to say,but they are not the same.The latter is the semi-reduced form of the former.
| Quote: | | LiAlH4 or hydrogenation can be used, but the hydrogenation workup is going to suck. |
It would be better if Red-Al is used for the reduction,since karlos is an expert with it.
Quote: Originally posted by zed  |
Working with cyanide isn't fun, but it looks like Benzaldehyde Cyanohydrin, may be commercially available |
I had an idea yesterday.could mandelonitrile be obtained from amygdalin ,or even better,from prunasin ?.I remember a member talking about the
extraction of amygdalin from apple seeds.
see this http://chemistry.stackexchange.com/questions/24528/how-do-i-...
and this http://eprints.whiterose.ac.uk/83873/2/Bolarinwa.pdf
[Edited on 15-10-2015 by CuReUS]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
First of all, I would like to thank all of you for the great ideas thrown in, the useful references and your contributions in general! It really gave
me a lot of inspirations, very appreciated.
| Quote: | | That´s why I stay away for the moment from acetophenone as a precursor. But if I wanted any specific isomer, then it would be the educt/starting
material of my choice. |
| Quote: | | Karlos,how do you plan to do the enantioselective synthesis ? will you use a chiral reagent ? |
I would like to use yeast, more specific their ADH enzyme, on it, the reduction of halo- or azidoacetophenone is reported in the literature. Also, I
really like working with yeast, except of the messy workup.
I really like the smell of yeast, its non-toxic, and a cheap reagent. Too bad that other microorganism for reduction of alcohols are so hard to get,
e.g. Rhodococcus and Candida species which are useful here.
Maybe I will try it with an chiral borane reagent derived from an aminoacid?
| Quote: | | Now I know that the chloroform is essential for this reaction, but it would be much better if it can be substituted with another solvent.
|
| Quote: | | I suggested using DMF in the previous page. |
Would it work, I would guess the needed chloride for the hexaminium chloride would be missing?
Do you have a reference for that, that would be great?
Thank you in advance!
| Quote: | | the difficulty in obtaining reagents used to form the epoxide, preferably mCPBA, which isn't too hard to make (it's on orgsyn) but it's not the sort
of thing you can buy at the store. |
| Quote: | | yes you are right.But karlos is not going to use mCPBA in the first place.There are many alternate,amateur friendly and OTC reagents to make the
epoxide. |
Well yes, I wanted to use H2O2 with MeCN, but it turned out I hadn´t any MeCN, I thought I had, but looking through my chemical cabinet I found there
was not any.
So unfortunately the epoxide synthesis has to wait...
| Quote: | | LiAlH4 or hydrogenation can be used, but the hydrogenation workup is going to suck. |
| Quote: | | It would be better if Red-Al is used for the reduction,since karlos is an expert with it. |
I think Red-Al is interchangeable in most reductions that can be performed with LiAlH4.
Also, my answer to this is probably off topic, but thank you for calling me an expert with Red-Al, I think of myself just as a novice in working with
this stuff  But thank you for the compliment! But thank you for the compliment!
Quote: Originally posted by zed  |
Working with cyanide isn't fun, but it looks like Benzaldehyde Cyanohydrin, may be commercially available[/rquote]
I had an idea yesterday.could mandelonitrile be obtained from amygdalin ,or even better,from prunasin ?.I remember a member talking about the
extraction of amygdalin from apple seeds.
see this. |
Well, it is commercially available but not that cheap.
But then again, phenethanolamine is also commercially available and cheaper.
I made recently a bit 2-nitrophenylethanol from benzaldehyde and nitromethan, catalysed with triethylamine. It was an 0,2mol (of benzaldehyde) batch,
and it gave after Zn/HCOOH reduction only 12g of phenethanolamine as HCl salt.
But anyway, I would like to try other methods out so I will continue my experiments.
Another idea came to my mind: I am a bit bankrupt at the time, so when I searched through my chemicals (when i was looking for the MeCN/acetonitril
for styrene epoxide synthesis) I stumbled over NaN3 and thought it would be a good reagent for use here.
I would like to make the halohydrin, bromo, or chloro, and then react it with the sodium azide to give the azidoalcohols which can be reduced with
NaBH4 to phenethanolamine.
That would be an option, wouldn´t it?
(Also, sorry for fucking up the quotation, I hope it is still readable and clear who said what?)
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
| Quote: | Well yes, I wanted to use H2O2 with MeCN, but it turned out I hadn´t any MeCN, I thought I had, but looking through my chemical cabinet I found there
was not any.
So unfortunately the epoxide synthesis has to wait... |
no need of MeCN,see this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=7641
nicodem talked a lot about OTC methods for making the epoxide
http://www.sciencemadness.org/talk/viewthread.php?tid=15284#...
The TCCA method he posted goes via the chlorohydrin,but I think the oxone/acetone method is more interesting.
| Quote: | I stumbled over NaN3 and thought it would be a good reagent for use here.
I would like to make the halohydrin, bromo, or chloro, and then react it with the sodium azide to give the azidoalcohols which can be reduced with
NaBH4 to phenethanolamine.That would be an option, wouldn´t it? |
I think it will.Azide ion is a better nucleophile than amine without the inherent naughtiness of giving tri-alkylated products. Read the chapter on
azides in "total synthesis 2" by strike.Its seems the azide can be reduced to amine just by adding Mg or Ca to it.
as for DMF,I suggested it because it was found that the yield for a gabriel reaction could be increased if DMF was used.Since you talked about wanting
to use pthalimide(from the german paper),I thought it would be helpful if I mentioned it.
[Edited on 18-10-2015 by CuReUS]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
*facepalm* Bad day at the library. I stand corrected. Carry on, gentlemen.
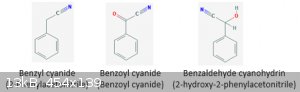
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Ethane-yl — ethyl.
Ethanoic-yl — ethanoyl.
Benzoic-yl — benzoyl.
Product of condensation of benzaldehyde with cyanide is benzaldehyde cyanohydrin.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Ok CuReUS, I thougt you´ve meant DMF for the Delepine, not Gabriel synthesis. Thank you for clearing that up.
I guess I will order some succinimide and try that too.
I tried the TCCA route to the chlorohydrin too, and it worked well enough.
But I will make the azide from the bromohydrin I think, more expensive but a better leaving group for making the azide(which itself is not that
cheap).
So many roads to phenethanolamine, that is really some fun in the lab!
@Praxichys: You can remember it more easy by thinking about the -hydrin as an addition product of water and cyanide/halogen, two carbons apart from
each other.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
But I will make the azide from the bromohydrin I think, more expensive but a better leaving group for making the azide(which itself is not that
cheap).
|
Yes,I was going to suggest the same thing.All the papers I have read till now use the bromo compound to make the azide.
But I have one question. Is there a chance of any intramolecular cyclization between the azide ion and the adjacent OH ?
(I don't think so,since azide ion has negative charges on both the terminal N atoms,but still ? )
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
Hello,
I want to dig up this thread because the discussion is very interesting. I myslef had biiiig problems to reduce nitroalcohol with metal/H+ or CTH !!!
I think the main problem can comes from dehydration to nitrostyrene in acidic environement.
I think you can open the styrene oxide on the right side with a strongly basic nucleophile such as NaNH2. The azide will also attack to the right side
and is naturally easy to convert to amine by any method. Azide can also do the SN2 behind the bromo of the NBS oxydation product of styrene in water.
I know the oxyamination but requires the exceedingly dangerous Os+8 compound.
You can start with protected glycine, then friedel craft acylation (one pot after chlorination), after reduction of carbonyl with NaBH4 the amino is
deprotected. The special part is choosing the most suitable protecting group :TFA support strong lewis acid and is easily cleaved with weak base,
carbamate are well known protecting groups but I dont know whether they survive AlCl3, maybe weaker lewis acid such as SnCl4 ? Any idee ?
You can also condense commercial mandelic acid or mandelate with ammonia and reduce the amide to target.
Other ideas are welcome.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
It's just interesting to watch that few people actualy have read the thread and papers in it (because every question is already answered here).
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I have the urge to report back about how it is going.
Actually I have decided to use gabriel/delepine like reactions and have purchased succhinimide, hexamine, and saccharin. I still have to wait for one
or two reagents for each route to arrive, but I was successfull in synthesising the epoxide(H2O2 in EtOH and MeCN). Also I prepared a few grams of the
bromohydrin (with NBS in DMSO) of styrene.
When the reagents I am awaiting come to my door, I will try to post a writeup about the synthesis of the adduct which synthesis works best.
I have abadoned the idea of using the azide, I mean, it´s convenient, but I would really prefer to make the title compound without any reduction.
At the moment I had much fun with this synthesis, actually I am cleaning a batch of my last HCOOH/Zn reduction. I hate this crap that is also produced
and lowers the yield!
But it is easy to clean the final salt.
Anyone who would like to attempt the synthesis of this compound via nitroalcohol, please use another reducing agent as Zn and formic acid!
I would like to try sodium amalgam as reducing agent once, have anything to make it, but I think it is a bit dangerous for me.
Don´t know if thats realistic.
But for know, styrene is intriguing me the most. As soon as my reagents arrive, I will report back.
Mandelic acid is something I don´t consider useful for that synthesis.
Maybe R-Mandelonitrile made with HCN from benzaldehyde with the oxynitrilase from almond meal, but any other way, no, not really. Reducing agents are
expensive and even if I have access to many of them, I would like to prepare this simple compound without any reduction.
Thanks to everyone who parcipitated here! I appreciate it very much, you all gave me very helpful ideas and other input!
Thats why I like this community so much 
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I can't understand whether Zn-HCCOH is a nice way to reduce the nitroalcohol or a messy one.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Well, it is a messy one, but easy to carry out and the reagents are very cheap.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Is it ?
http://www.sciencemadness.org/talk/viewthread.php?tid=34833#...
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yes, in a larger, preparative scale it certainly is.
Mostly because of the large solvent volumes needed, and also the Zn salts, which can be vac-filtered, or, (some substrates are not suited for this)
with large amounts of NaOH you can prepare the soluble sodium zincate salt, which eats a lot of NaOH and consequently increases the volume.
Otherwise, the reagents are quite cheap, but the solvents needed are not.'
Have you done a Zn/HCOOH reduction in a scale of like a mole? It sucks to work with this mess.
I would likely have another option.
But for the moment I´ve decided I will do reduction-free synthesis routes.
Even if they are not as high yielding, the delepine like reactions are very charming in my opinion. Very clean, small solvent volume, easy recycling
of the solvents and cheap reagents (succinimide, saccharine, hexamine are so cheap! The disadvantages are, hexamine needs TCM, saccharin DMF, but only
a tiny amount, but succinimide does not, only some alcohol and pyridine which are both cheap).
I guess my Pd/C is of a very bad quality. Otherwise I would go this way. I don´t want to waste my MeNO2 for this stuff at the moment, so I am going
the styrene routes.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Didn't catch where you had problems with your Pd/C. Nitriles do poison some Catalysts.
Also, freshly prepared Catalysts are usually more active.
|
|
|
| Pages:
1
2
3 |