| Pages:
1
2
3 |
Upsilon
Hazard to Others
  
Posts: 392
Registered: 6-10-2013
Member Is Offline
Mood: No Mood
|
|
Sulfur as an oxidizing agent
I'm trying out possible ways to create hydrogen sulfide gas other than the traditional sulfide salt + acid reaction. My thoughts went to seeking out
an anion that is a less powerful oxidizer than sulfur. This way, a solution of the acid of the anion would be oxidized by the sulfur, leading to the
sulfur replacing the anion.
The issue is that sulfur is a very weak oxidant. I even tried sulfur in aqueous citric acid but nothing happened. It must be possible with some acid,
though, because it is listed as a viable half-reaction in tables of reduction potentials:
S(s) + 2H+ + 2e- -> H2S(g)
Any suggestions here?
|
|
|
Brom
Hazard to Self
 
Posts: 94
Registered: 19-7-2015
Member Is Offline
Mood: No Mood
|
|
Burn a mixture of aluminum powder and sulfur which will leave you with aluminum sulfide. Adding water to the aluminum sulfide generates H2S.
|
|
|
morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Reaction of NaOH with sulphur produce Na2Sn
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Burning paraffine wax with S in closed container will generate H2S and stinky oils...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Consider oxalic acid and sulfur:
HOOCCOOH + S => H2S + 2CO2
The oxalic acid acts as both the reducing agent and proton source. It's theoretical, I don't know if it would work for sure. I trust you understand
the dangers of H2S!!!
[Edited on 27-9-2015 by deltaH]
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Just to elaborate on this, this is an older method I have seen in lab manuals from the turn of the last century or so. This always intrigued me since
the sulfur is acting as an oxidizing agent, and what if I wanted to go further to CS<sub>2</sub>? Anyway, usually a mixture of sulfur and
paraffin is heated in a test tube with an alcohol lamp, a piece of glass wool is placed atop the mixture to prevent spattering and the gas is lead
away through a piece of glass tubing inserted into a rubber stopper (or better yet old-school cork stopper coated in waterglass).
I am surprised that no one has mentioned it so far, but take care. I don't know what your person skill level or knowledge base entails so this may
come off as something you have seen again and again but H<sub>2</sub>S is nasty stuff. Kills your sense of smell, and then it can kill
you. Don't rely on odor, make some lead acetate strips or the like to act as a visual warning and get a respirator (although not many are rated for
continuous use).
[Edited on 9/27/2015 by BromicAcid]
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
HF, arsenic and now H2S.
What are you making, upsilon?
|
|
|
Upsilon
Hazard to Others
  
Posts: 392
Registered: 6-10-2013
Member Is Offline
Mood: No Mood
|
|
deltaH, that was what I was going to try next. Right now I am testing tiny amounts outdoors before I start actually trying to use it as a reagent.
I'll come back with results tomorrow.
j_sum1, I decided against HF and won't be trying anything with it for a long while, if ever. Arsenic for an element collection. H2S, well, I did have
a use for it in mind but I forgot about it ever since I started thinking of ways to actually make it. I'll remember soon enough.
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
I suggest that you make chemistry journal, and write everything you ever learn, hypothesize, do or plan to do chemistry-wise in it. It would probably
make your life as a chemist much easier, and hey—you may even learn a thing or two and possibly teach others as well.
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The big question is what reaction you need H2S for LA:Upsilon, as j_sum1 asked.
You might be going about the Real reaction all lizard-fisted, and may not need H2S at all for the Actual reaction you intend.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by LA:Velzee  | | I suggest that you make chemistry journal, and write everything you ever learn, hypothesize, do or plan to do chemistry-wise in it. It would probably
make your life as a chemist much easier, and hey—you may even learn a thing or two and possibly teach others as well. |
Take it from a lizard alien deltaH, as you won't take it from mere humans.
Write your ideas down, post here, video or voice record it.
Not all of will be worthwhile, yet a lot of what spouts from your brain is Good stuff, and you should record it in some way.
|
|
|
Upsilon
Hazard to Others
  
Posts: 392
Registered: 6-10-2013
Member Is Offline
Mood: No Mood
|
|
Just tried elemental sulfur in 100mL of a 1M oxalic acid solution, no reaction evident. This is actually surprising, since the table of reduction
potentials suggests that sulfur is the most favorable to be reduced in the hypothesized reaction, leaving oxalate to be oxidized to carbon dioxide.
[Edited on 28-9-2015 by Upsilon]
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
This AL is ignorant. I request the help of you humans:
How can I get NaOH to react with elemental sulfur to produce Na2Sn, as @morsagh suggested?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by Upsilon  | Just tried elemental sulfur in 100mL of a 1M oxalic acid solution, no reaction evident. This is actually surprising, since the table of reduction
potentials suggests that sulfur is the most favorable to be reduced in the hypothesized reaction, leaving oxalate to be oxidized to carbon dioxide.
[Edited on 28-9-2015 by Upsilon] |
I'm happy you tried it and I'm not that surprised. The problem might be the insolubility of sulfur and ionic nature of oxalic acid in water.
You might have success by melting oxalic acid with sulfur in a test tube. Again, be careful please... use very small amounts.
In short, the reaction is thermodynamically favourable, BUT that says nothing about kinetic barriers. However, kinetic barriers can be overcome, heat
is the usual method, failing that or when there are selectivity issues, one can try to employ a catalyst to help speed things up.
Good luck and thanks for trying it out!
[Edited on 28-9-2015 by deltaH]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Use the action of acid on Iron (II) sulfide. I assume your aversion to metal sulfides is caused by their unavailability and/or violence of
manufacture.
Heat a well-mixed, stoicheometric (1:1 molar) pile of iron filings and powdered sulfur with a torch. The reaction to FeS2 is nice and gentle; it looks
as if the pile is slowly smoldering. When cool, grind into a fine power and remove any unreacted iron with a magnet. Store in a stoppered bottle. If
you do this in a small tin can, you can manufacture >100g each time, enough for over 1mol H2S.
Exposure of the sulfide to acids will release H2S. Strong mineral acids are preferred but it will work with many organic acids as well. Be careful.
H2S kills your sense of smell at ca. 100ppm, causes unconsciousness at ca. 300ppm, and is rapidly fatal at 1000ppm. (Keep in mind 1000ppm is 0.1wt%)
[Edited on 28-9-2015 by Praxichys]
|
|
|
Upsilon
Hazard to Others
  
Posts: 392
Registered: 6-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  | Quote: Originally posted by Upsilon  | Just tried elemental sulfur in 100mL of a 1M oxalic acid solution, no reaction evident. This is actually surprising, since the table of reduction
potentials suggests that sulfur is the most favorable to be reduced in the hypothesized reaction, leaving oxalate to be oxidized to carbon dioxide.
[Edited on 28-9-2015 by Upsilon] |
I'm happy you tried it and I'm not that surprised. The problem might be the insolubility of sulfur and ionic nature of oxalic acid in water.
You might have success by melting oxalic acid with sulfur in a test tube. Again, be careful please... use very small amounts.
In short, the reaction is thermodynamically favourable, BUT that says nothing about kinetic barriers. However, kinetic barriers can be overcome, heat
is the usual method, failing that or when there are selectivity issues, one can try to employ a catalyst to help speed things up.
Good luck and thanks for trying it out!
[Edited on 28-9-2015 by deltaH] |
Looks like I'll be doing that then. Oxalic acid and sulfur have conveniently similar melting points so it shouldn't be too much of a hassle. I'll try
it out sometime this week.
Praxichys, that may very well be a better method, but I'm going to try this out anyway and see if it's more efficient. If not, then oh well, that's
what experimentation is all about. That, and I don't have any iron filings on hand 
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
They may have similar melting points, but not sure if they will be miscible when molten. That said and with no water, oxalic acid can't ionise easily
and in its unionised form it is symmetrical and so non-polar, so maybe it works. Experimentation tells all. At the very least, the higher temperatures
should help a lot!
I look forward to your results 
[Edited on 28-9-2015 by deltaH]
|
|
|
Brom
Hazard to Self
 
Posts: 94
Registered: 19-7-2015
Member Is Offline
Mood: No Mood
|
|
Do you not have powdered aluminum? If so the Al2S3 route is an extremely simple path to H2S
|
|
|
Upsilon
Hazard to Others
  
Posts: 392
Registered: 6-10-2013
Member Is Offline
Mood: No Mood
|
|
I considered that as well, but aluminum powder isn't exactly cheap. I would argue that even requiring an acid to free the H2S, the iron sulfide route
is still cheaper than using aluminum powder.
|
|
|
Brom
Hazard to Self
 
Posts: 94
Registered: 19-7-2015
Member Is Offline
Mood: No Mood
|
|
Ok I understand the quote button now. I was having trouble with the edit feature. After I would edit I could not post it after it was fixed. Thanks
for the help. Sorry for not being so good at the computer thing. I guess I'm getting old
|
|
|
Brom
Hazard to Self
 
Posts: 94
Registered: 19-7-2015
Member Is Offline
Mood: No Mood
|
|
I figured it all out now. Thanks again for the help
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Doesn't oxalic acid decompose on heating to release carbon monoxide?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
That's an interesting point, it does decompose to carbon monoxide, carbon dioxide and water vapour between 150°C - 200°C (see the thermogravimetric
plot below from http://www.azom.com/article.aspx?ArticleID=3024) , so again, please be careful. Even if unsuccessful, an odourless toxic gas will [nevertheless]
be evolved.
I guess success will depend on which has the faster kinetics at those temperatures: oxidation or the [self] decomposition of oxalic acid. Reminds me
of the behaviour of hydrogen peroxide where there is a similar competition, albeit in a reversed role as oxidising agent (oxalic acid being a reducing
agent).
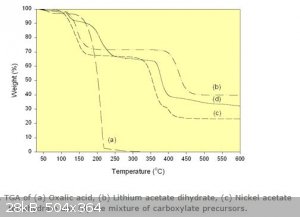
[Edited on 29-9-2015 by deltaH]
|
|
|
AJKOER2
Harmless

Posts: 2
Registered: 29-9-2015
Member Is Offline
Mood: No Mood
|
|
Here is another aqueous approach, to quote Atomistry on H2S, which contains dated historical accounts (link: http://sulphur.atomistry.com/hydrogen_sulphide.html ):
"(b) Sulphur can also be reduced to hydrogen sulphide at the ordinary temperature if "nascent" hydrogen is used; thus, powdered sulphur yields some
hydrogen sulphide if treated with aluminium, tin, iron or zinc and hydrochloric acid, the result being improved by the additional presence of acetic
acid or alcohol, which will increase the solubility of the sulphur. The reduction can also be effected electrolytically by having powdered sulphur in
contact with a platinum cathode immersed in dilute acid, e.g. hydrochloric acid."
My updated rendition of the pathway is electro-chemical based on the so called Aluminum Sulfur battery (see, for example, "Aluminum and sulfur
electrochemical batteries and cells", US patent 5,413,881, link: http://www.google.com/patents/US5413881 ). The cell reaction on which I am suggesting a modification is given generally as:
2 Al + 3S + 3OH- + 3H2 O → 2Al(OH)3 + 3HS- ; Ecell =1.8 V
Now, if one adds acid (say HCl) to both sides of the equation, with sufficient acid, one could have, for example:
HCl + NaHS → NaCl + H2S (g)
--------------------------------------
[Edit] A side comment is that all of the cited metals (aluminium, tin, iron and zinc) associated historically with "nascent hydrogen" formation above
have a possibly interestingly corresponding electro-chemical basis as I argued above. Specifically, for example, existing metal-air batteries include
aluminium-air, tin-air, iron-air and zinc-air.
[Edited on 30-9-2015 by AJKOER2]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Why is it AJKOER2 and not good old AJKOER ?
Your link-finding abilities are legendary : all fascinating and almost 100% relevant.
|
|
|
| Pages:
1
2
3 |