Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
500 ml copper flask
This is something I've wanted for a long time. It will be useful when highly caustic solutions must be heated to reflux.
I could not find one of these offered by any chemical supply house. I did find a vendor of copper spheres for decorative use. To this I silver
soldered a 3/4" x 1" copper reducer for copper pipe. A one-hole rubber stopper provides a connection to my 19/22 condenser.
http://www.necopperworks.com/copperballs.html
In the photo I'm testing it with 350 ml of 10% NaOH at reflux. I found no leaks. Wall thickness is 0.032"(0.81 mm), which seems adequate for
labwork. Ball diameter is 4".

[Edited on 5-7-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cool purchase Magpie !
Is they soldered joints halfway up and at the neck joint ?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Very cool! Was there a trick to drilling the hole?
I could see this coming in handy for a lot of reactions.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
nice work Magpie, i might have to try my hand at making one of those also. just wish i had some sheet flashing that was a bit thicker than my current
stock.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The biggest trick in drilling any hole is to identify the precise spot where the centre should be, then bash that exact spot with a centre punch to
make an indentation.
Your first drill will be small diameter (aka pilot drill) and will nicely go into that tiny depression you made with the punch.
After that, just keep increasing the drill size and drill it again to make biger and bigger holes, also centred on that same spot (within reason).
[Edited on 5-7-2015 by aga]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
It was not tricky at all to drill the hole - the vendor drilled it! I requested a 1.00" hole. The hole fit the reducer perfectly so soldering in the
reducer was easy.
From there on it became a comedy of errors. The sphere is made by soldering two hemispheres, the seam being barely visible. My machinist thought
that it was soft solder and I moaned. I agreed as it would be cheapest and all that is needed for decorative use. It was the weekend so we couldn't
call the vendor and ask. Being impatient old fools we forged ahead. He melted the seam a little then declared " by golly it is silver solder!" By
then it was too late and he had to resolder it with new silver solder.
It looked a mess with all the oxide and flux marks so I asked if he could remove the marks. So he placed it in his lathe and using a flapper wheel
tool soon had it shiny again. But this turned the perfect circle of the reducer opening to a rounded triangle. Luckily I had a 1 1/8" dowel. After
inserting the dowel I gently squeezed the reducer opening with my vice until it was very nearly circular again.
He said "next time we will fill the flask over half full with water and thereby preserve the original seam. There never will be a next time. My
creations are "one-offs" - does he think I am made of money?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I think it's awesome.
You can do the exact same thing with stainless if anyone is so inclined. There are lot's of solders/flux-s around that work just as easily as with
copper.
Very cool flask Magpie!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
@ aga: the center-punch and pilot hole are dead on. but you have to pick a reasonable size drill next( not super critical, but think first). it's
one thing if it is thick steel, and another with thin, soft and unsupported copper. it is a soft metal ( especially after annealing) unless work
hardened, and will wad up, bend and bind.
pick wrong and you may see
>drill bit will tumble while trying to get a bite and make a triangular opening
>one side catches *hard; stuck on a copper splinter, tries to twist the piece/deform the opening.
- too big of a jump in sizes, copper deforms without support, the amount of metal that is trying to be taken off usually exceeds the coppers ability
to hold rigid at that thickness. I have had this cause drift also, as the thinned section mushrooms downward before cutting, followed by a sharp
grab.
-too small of a jump, and you are riding more on the shoulder of the conical tip than the apex, and it can cause problems centering. some times leave
a beveled edge on one side, if it drifts to find a good bite. but the amount of metal being taken off is better supported by the sheet.
- a reasonable size difference ensures good centering and less of a chance of deformation from binding.
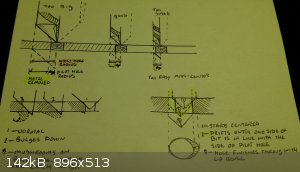
*** not always the case, just my observations. I love copper work***
@ Magpie: did he use a whetted newspaper to wipe the solder smooth? a lot of sheet metal guys will put it on thick and then wipe it hot, to remove
excess. if it is still messy and clamped in place, wired bound in place or in a vice( basically fixed against separating at heat), you can re-heat
the drips and wipe them off, or at least really thin. wet auto sandpaper will take care of the solder smear quickly, 600grit is a good start for me.
*heavily edited to avoid misinformation from personal observation, while using more than one brand/type of bits on same projects*
[Edited on 6-7-2015 by violet sin]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
No, using an acetylene torch he just melted the wire onto the seam as I rotated the sphere a little at a time. After it had cooled he poured it over
half full of paint thinner to check for leaks.
He is not a copper worker per se, but has a wealth of experience in welding all sorts of metals over a long career. He's welded zirconium,
titanium, and tantalum. He has his own machine shop and at one time supervised 17 machinists. He's semi-retired and takes on my odd jobs with a
minimum of fuss and paperwork.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's a better picture of the flask. The blue liquid is the 350 ml of 10% NaOH after refluxing in the flask for 1/2 hour or so. Copper is so
conductive that I had to cover it with a fiberglass blanket to get it up to boiling.

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Magpie is the blue liquid intentional, or formed as a result of dissolving residual copper oxide on the inside of the flask to form
copper cuprate?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Not intentional. Must be sodium cuprate.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
These semispheres would be nice for homemade heating mantles.
Fear is what you get when caution wasn't enough.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Very cool. I'd been looking into making a copper still for a Swartz reaction. It tends to produce some HF and destroys glass equipment, but I also
need to be able to fractionate.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
in regards to they professional you had do the work,
AWESOME!!! sounds like a guy I would love to know/live near, and ask questions frequently... the water safe silver solder I have been using works
just fine by propane torch, but I doubt it would be all that chemically inert. he must have used the high silver content higher temp hard solder.
this guy, https://www.youtube.com/watch?v=z1elySHFnE4 , wiping in potassium fluoride flux with bare hands... while soldering Cu - steel.
I actually have two nice chunks of Zr(705) plate waiting for the right projects,~ 4"x6"x1". though all my Ta is in thin strips, a couple pounds, but
practically useless for fabrication. always dreamed of having a machine shop my self
[Edited on 6-7-2015 by violet sin]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The final (and successful) test of the flask was reflux of 157g of 68wt% NaOH for nearly an hour. There was mild bumping even though I was
magnetically stirring.
Attachment: phpACAyY1 (74kB)
This file has been downloaded 999 times
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
So no blue his time, outstanding, I'd say that's quite a nice addition to any lab's stockpile. Way to go. What would you say was the rough price of
your project?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Next time you see him mutter Mandrel under your breath, just loud enough for him to almost hear 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by violet sin  | So no blue his time, outstanding, I'd say that's quite a nice addition to any lab's stockpile. Way to go. What would you say was the rough price of
your project?
|
There was just a little blue color, and no oxide flakes. The rubber stopper and ptfe oval stir bar were unaffected.
The flask itself with one free hole is $29 as you can see from the vendor's website. His shipping (UPS) was an exorbitant $17.
The reducer is ~$4 at your local hardware store. I cut back the 3/4" end to about 1/8" before welding so there would be very little excess extending
into the flask.
My welding cost was $35. It could be cheaper if you learn from my mistakes.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Texium
|
Thread Moved
22-11-2023 at 19:33 |