Tensai
Harmless

Posts: 1
Registered: 30-4-2015
Member Is Offline
Mood: No Mood
|
|
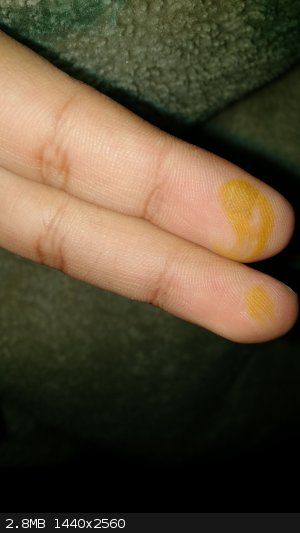
H2SO4/ HNO3 mix on my hand
Hello,
How are you doing, chemists?
Actually, I got into an accident 4 days ago in Organic Lab while mixing H2SO4 and HNO3, the accident happened when I took out the thermometer and
touched it with my two fingers while still having the mixture in it,
The tempreture was about 10-20 celsius
There is a still yellow scar around my finger and I am afraid it won't vanish that easily. I asked my lab instructor, and he told me that it is okay
and it will go away soon. But I am still worried-.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
The yellow color will fade and I doubt you will have a permanent scar. I've seen way worse end up perfectly fine.
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
I'm new to chemistry as a hobby and have already stained my hands more times than I care to admit;) WFNA, RFNA and just last week silver nitrate
solution.
Don't worry it will fade away soon
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
The yellow stain is caused by the nitric acid. As everyone says, don't worry, it will go away.
Of the 3 common mineral acids, the order in which to worry about skin contact, from least to most worry, is HCL -> HNO3 -> H2SO4, and HF is off
scale. In any case, if you get some on you, wash it off immediately. And don't under any circumstances get any of these in your eyes.
Any other SF Bay chemists?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The yellow stuff will peel off in a few days. If you're the kind of person who enjoys peeling sunburns, you'll have fun with it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
The other day I showed my wife what anhydrous nitric acid will do to a nitrile glove.
Needless to say she is now even more terrified.
On another note, McMaster has very cheap Viton o-rings for when you have to replace the thermometer adapter o-ring. 
[Edited on 30-4-2015 by Loptr]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Very useful loptr. I think I will order some.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The yellow comes from tiny amount formation of picric acid from amino acid tryptophan.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I find that hard to believe. A lot of nitrated things are yellow, not just picric acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Make sure you get the cheap ones, since they do sell some expensive ones. I received 100 for probably about $7 total.
[Edited on 30-4-2015 by Loptr]
[Edited on 30-4-2015 by Loptr]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
It is called xanthoproteic reaction and forms xanthoproteic acid upon reaction of aromatic ring containing amino-acids in proteins...like tryptophan,
tyrosin, phenylalanine. The easiest nitrated one is tyrosin.
The yellow colour turns orange in basic media what is very common with dinitrophenol and trinitrophenol related compounds.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
tahallium
Harmless

Posts: 41
Registered: 24-2-2020
Location: Tunisia
Member Is Offline
|
|
I spilled sodium chromate solution (bleach and chromium hydroxide method) on my hand two day ago I washed my hands immediately and I don't know what
should I do
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by tahallium  | | I spilled sodium chromate solution (bleach and chromium hydroxide method) on my hand two day ago I washed my hands immediately and I don't know what
should I do |
How much did you get on you? Dermal contact with chromate while not ideal is the least concerning exposure pathway. It can lead to dermatitis and
sensitisation after reaped exposure. Do you have any irritation on the stop where you were exposed? Exposure for longer durations or to larger amounts
can lead to ulceration. Ingestion or inhalation has far worse consequences. Did you cary this out in a well ventilated area? I imagine you had some Cl
to deal with, but I don't know how fast this reaction would proceed..... Probably best to review your choice of PPE before trying this one again.
|
|
|
Texium
|
Thread Moved
28-4-2020 at 06:45 |
Fery
International Hazard
    
Posts: 1016
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Tensai - it will disappear. Decades ago I used conc. HNO3 to get rid of skin warts, very efficient, though I do not suggest this method to others
(risk of exposing for too long).
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by Fery  | | Tensai - it will disappear. Decades ago I used conc. HNO3 to get rid of skin warts, very efficient, though I do not suggest this method to others
(risk of exposing for too long). |
Oh man... ;D how did it go? Sometime ago I thought should I try conc. NaOH on my wart but left this idea.
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by Fery  | | Tensai - it will disappear. Decades ago I used conc. HNO3 to get rid of skin warts, very efficient, though I do not suggest this method to others
(risk of exposing for too long). |
Laboratory assistant from my former school use silver nitrate for this  . .
|
|
|
Fery
International Hazard
    
Posts: 1016
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Bedlasky - yes, Lapis is safer but must be applied locally for few days/weeks. HNO3 only once was enough.
Mackolol - it was very efficient, just if I overexposed then it slightly burned for some time but not too much. Wart always disappeared.
Today I suggest more gently methods like Verrumal available in my country (fluorouracyl which is cytostatic and blocks virus replication+ salicylic
acid in dermatolytical concentration which disrupts and dissolves keratine and keratinocytes) - requires approx 1 month of local treatment. Or liquid
nitrogen. Concentrated formic acid too (recently I saw 1 new antiwart medical IIRC of 5 ml which price was 3 fold of 1 L of 85% formic acid from chem
supplier).
There is usually a problem that the virus persists somewhere in the human body so even if you win against 1 wart there will be soon created another
one at different location 
If you have not only 1 wart but a lot of them, there could be probably weak immunity (or very aggressive virus).
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
So you produced leather nitrate.
Minor spills are not that biggie, they dissipate in few days. I've got mine too.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Fery  |
There is usually a problem that the virus persists somewhere in the human body so even if you win against 1 wart there will be soon created another
one at different location 
If you have not only 1 wart but a lot of them, there could be probably weak immunity (or very aggressive virus). |
These papilloma viruses do not enter the body, and therefore don't persist inside the body. They spread via skin lost from warts, they are very stable
outside the body and stay infectious for a very long time.
If you had warts on for example your hands and they keep coming back on different spots... Maybe you should take a day or two off and give your house
a really proper cleaning.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Lol, first time? You’re fine.
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
Like the others said, you will be fine. As a side note, just getting near 68% nitric acid was enough to almost destroy one of my nitrile gloves I was
wearing, and there wasn't even direct contact 
Also, 30% hydrogen peroxide can leave scary looking white patches. I believe it is oxidizing / burning your skin. It happened to me once on my finger,
but it didn't hurt and it went away in a few hours.
-------------------------------------------------------------------------------
ChemTalk
Chemistry Experiments
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I've noted that 30% H2O2 actually stings a lot when it gets onto skin. My usual way of exposure is handling a bottle of it, and when I pour or handle
the cap, there will always be that one damn droplet that flows down the side of the bottle, and once you pick it up, you smear it on your fingers, and
within a minute you feel that stingy feeling and see white patches on your skin.
Unless I'm handling something toxic or bioaccumulating stuff, I usually just go bare handed, because I've melted my fair share of all kinds of gloves
and slipped some glassware due to bad grip. If it's too bad, I look through compatibilities before touching it. A good practice for limiting
contamination is to immediately wipe all drops, leaks and spills and the neck of a flask or bottle when handling them.
|
|
|
Xanax
Hazard to Self
 
Posts: 54
Registered: 28-8-2003
Location: Sweden
Member Is Offline
Mood: Curious
|
|
Once I was trying to remove my fingerprints with nitric acid, but the finger tops healed and the prints are still there.
But I Have an ugly burn scar on my arm, from nitric acid...

[Edited on 2021-5-18 by Xanax]
|
|
|
Aloesci
Harmless

Posts: 28
Registered: 7-4-2018
Member Is Offline
Mood: No Mood
|
|
My Conc. Nitric acid yellow marks stayed on my hands for about two weeks!
I was kinda happy that I'd made concentrated nitric so i didn't take enough care when dissasembling the glassware. (no gloves because i didnt want
flaming nitrile burning my skin)
I think it depends how long it was on your skin and how many layers deep it penetrated.
|
|
|