| Pages:
1
2 |
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Synthesis of Oxygen Absorbing Crystal (from cobalt)
Ok guys, that new crystal they discovered from cobalt is crazy useful, some number liters (prolly a few) can suck up all the oxygen out of a room and
can hold three times as much as a high pressure oxygen tank.
so obviously i want to make it.
The name of it is:
"*If you must know, the chemical name of the salt is written out as {(bpbp)Co2II(NO3)}2(NH2bdc)2 * 2H2O, where “bpbp” stands for
2,6-bis(N,N-bis(2-pyridylmethyl)-aminomethyl)-4-tert-butylphenolato, and “NH2bdc2” stands for 2-amino-1,4-benzenedicarboxylato). Don’t ask us
how to pronounce all that."
If you can tell me how to make this... i will make it and provide pretty pictures and advice on my experience. And if i can make a lot, I will
certainly sell it to you. I have some experience with chemistry and hard determination to learn and accomplish but I start to get distant when the
names are longer than my attention span LOL
even if i need industrial equipment i have a shop to make all that in
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Are you referring to this?
http://pubs.rsc.org/en/Content/ArticleLanding/2014/SC/C4SC01...
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Yes it is! i saw a patent but was going to find a day off from work to rent it and do a bunch of research on it. Most of the time synthesis is written
out in patents, hopefully this one is too.
[Edited on 13-4-2015 by SupaVillain]
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
relevant username much?! don't make me regret this 
Edit(woelen): Cmos6667 asked me to remove downloadable file, due to copyright issues.
[Edited on 16-6-15 by woelen]
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
LOL i dont know of much evil i can do with oxygen crystals. These may prove to help much in firefighting to starve fires of oxygen and make safer and
cheaper oxygen tank setups for the firefighters.
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
right, that's what I thought! but seriously though, this could be useful for making inert environment for reactions
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by cmos6667  | | right, that's what I thought! but seriously though, this could be useful for making inert environment for reactions |
And this is what I thought as I read the abstract. I was looking into different methods for deoxygenating various solvents a while back, and this
might be something of interest.
EDIT: Also, possibly as a source of oxygen if the complex could readily release it in a controlled manner.
[Edited on 13-4-2015 by Loptr]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Might be a good media replacement for PSA-based oxygen concentrators, especially for generating near-100% oxygen. It could have applications in many
industries - imagine a self-regenerating low pressure oxygen tank. Long-lasting rebreathers, self-regenerating welding equipment, oxygen storage for
submersibles and hypoxic, high-altitude environments...
|
|
|
Dr.Bob
International Hazard
    
Posts: 2823
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
They show that they can release it simply by heating. The system does look great for both removing oxygen from gases for inert streams (like glove
boxes), as well as oxygen concentrators, like what old people with CPOD and emphysema use to breath.
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
it takes upwards of 24h to absorb oxygen and that oxygen is released upon heating, so I highly doubt your idea for fire extinction.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
HMMMMMM okay well that sucks that means it cant be used to knock people in a room. Well as far as the 24 hour figure for absorption... how much are we
talking???? I dont care if it takes 24 hours if over that span of time it could do a whole airgas truck of oxygen cylinders! Either way im sure i wont
care sinve its way cheaper than an oxygen concentrator and the whole setup
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
Here's what you could do: you know how cigarettes have a lot of tar and ash? That's because of incomplete combustion - increase the oxygen and voilà,
low tar cigarettes 
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cmos6667  | Here's what you could do: you know how cigarettes have a lot of tar and ash? That's because of incomplete combustion - increase the oxygen and voilà,
low tar cigarettes  |
I think this would cause the cigarette to burn vigorously even when you aren't sucking on it, rather than eliminate the tar. Still though, I imagine
it would be amusing to try.
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Besides the sigaret vigorously burning, also the nicotine would burn...
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
or maybe isolate the nicotine, then nitrate the hell out of the cellulose, then put the nicotine back
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
That's not how you make exploding cigarettes.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Replace the tar with volatile {(bpbp)Co2II(NO3)}2(NH2bdc)2 * 2H2O! That sounds like a great idea!
I would be amazed if someone could make this at home.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Im really confused here, the main chemical discussed is {(bpbp)Co2II(NO3)}2(NH2bdc)2 * 2H2O, but only synthesis for ones ending with 18, 12, 11, 15,
5H20's and a MeOH one are included. Are these more useful variations of the crystal? Which one is the most useful for oxygen purposes? Or am I
supposed to know how to further turn these things into {(bpbp)Co2II(NO3)}2(NH2bdc)2 * 2H2O????????
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
Pls halp
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
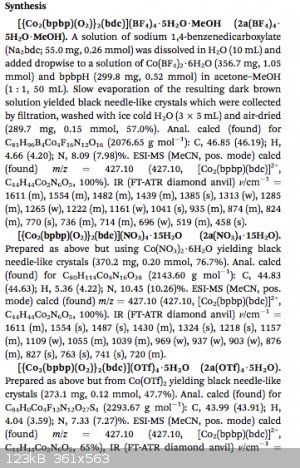
Supa Villan, a more detailed documentation of synth is on the last two pages of document. Here is the beginning of it. You are going to need bpbpH
which I think stands for bipyrrolidine, but I am not 100% sure. You could easily look it up, after all you have access nearly infinite information
right at your fingertips! 
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
My issue is that each of the bold text names on the synths are different compounds, unless Im wrong, I dont know which one to choose but I guess I'll
just go with the easiest one, they said also that two of them are slower to absorb oxygen than the rest.
At least cobalt nitrate hexhydrate is cheap and easy to obtain off of ebay.
|
|
|
Consequence
Harmless

Posts: 1
Registered: 16-4-2015
Member Is Offline
Mood: No Mood
|
|
Sorry I can't login as SupaVillain, password issues and not receiving emails within temp password....
anyways I've found the synthesis for bpbpH,(aka Hbpbp?) which is necessary for pretty much all the syntheses.
bpbpH SYNTHESIS
2,6-Bis{[bis(2-pyridylmethyl)amino]methyl}-4-tert-butylphenol,
Hbpbp
4-tert-Butylphenol (1.994 g, 0.0133 mol) and p-formaldehyde
(1.55 g, 0.0517 mol) were suspended in ethanol (25 cm3
). A
solution of N,N-bis(2-pyridylmethyl)amine (10.0 g, 0.0503 mol)
in H2O (50 cm3
) was added and the slightly yellow suspension
was stirred at reflux temperature for 3 d. The two-phase reaction
mixture was allowed to cool to room temperature and was
then partitioned between CH2Cl2 (200 cm3
) and H2O (100 cm3
).
The aqueous phase was extracted with CH2Cl2 (3 × 50 cm3
) and
the combined organic phase dried over anhydrous Na2SO4.
After removal of the solvent, the resulting brown oil was chromatographed
on a silica gel column using acetone as eluent
affording the crude product (5.86 g, 77% yield) as a slightly
yellow solid (m.p. = 122–126 8C). Recrystallization from diethyl
ether–light petroleum resulted in the pure product as white
crystals (60% relative to the crude product, m.p. = 123 8C). 1
H
NMR (250 MHz): d 1.27 (s, 9 H), 3.82 (s, 4 H), 3.90 (s, 8 H),
7.08 (m, 4 H), 7.19 (s, 2 H), 7.48–7.61 (m, 8 H), 8.52 (m, 4 H),
10.80 (s, 1 H). 13C-{1
H} NMR (62 MHz): d 31.57 (s), 33.85 (s),
55.12 (s), 59.88 (s), 121.81 (s), 122.84 (s), 123.16 (s), 125.85 (s),
136.33 (s), 140.79 (s), 148.83 (s), 153.43 (s), 159.39 (s) (Found:
C, 75.49; H, 7.04; N, 14.67. Calc. for C36H40N6O: C, 75.23; H,
6.96; N, 14.48%). FAB mass spectrum: m/z 572 (0.5, M1), 93
(100%, C5H4NCH2
1)
(from "Dinuclear iron(III)–metal(II) complexes as structural core models for
purple acid phosphatases †"
Morten Ghiladi, Christine J. McKenzie, Anke Meier, Annie K. Powell, Jens Ulstrup, and Sigrid Wocadlo
J. Chem. Soc., Dalton Trans., 1997, Pages 4011–4018)
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
I've recently started work on synthesizing one of these crystals, and I think that I've figured out a pretty good method. I have yet to test it since
I'm waiting on my distillation apparatus to arrive so I can make nitric acid, but the general idea of the synthesis is simple. The two more complex
chemicals required for the easiest synthesis in the document are disodium terephthalate, which can be made by the "PET to terephthalic acid" method by
Chromium, and cobalt nitrate, of which the synthesis is pretty obvious. I have some pieces of PET bottle cut up and ready to process, and I'll make
cobalt nitrate from cobalt carbonate as soon as I can make nitric acid. I'll be sure to report back here once I do it. A synthesis for a possibly
better crystal could be done by nitrating terephthalic acid and reducing the nitro group. I'll likely test both, but definitely the one with disodium
terephthalate first. EDIT: Forgot about the bpbpH! I'll look into that.
[Edited on 6-8-2015 by DFliyerz]
|
|
|
Mrinny
Harmless

Posts: 1
Registered: 15-12-2017
Member Is Offline
Mood: No Mood
|
|
Hey @DFliyerz
Any progress with the synthesis? How far did you come along? Did you stop midway? Any updates will be cool.
|
|
|
Xiomy
Harmless

Posts: 1
Registered: 20-1-2018
Member Is Offline
Mood: No Mood
|
|
Hello, I need to make the synthesis of these crystals in the university, all the information here has helped me a lot to know where to start, but I
would like to have more help from you if possible.
Thank you, good day.
|
|
|
| Pages:
1
2 |