| Pages:
1
..
37
38
39
40
41
..
104 |
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
The pool treatment tablets are either Calcium Hypochlorite or Trichloroisocyanuric Acid, and I think most are the latter.
I seem to recall reading somewhere about this. I think the reaction of the acid and the Hydrogen Peroxide evolves Singlet Oxygen, which is the
electronically excited state of O2. When it comes down off it's high, so to speak, it emits red light.
It's a pretty interesting reaction which I will have to try out one day.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
Quote: Originally posted by Argentum  | Something I've seen but can't find anywhere
When mixing H2O2 with pool water treatement pills it gave a red chemiluminiscence, am I right?
If I am, what is the reagent in the pills? (I think trichloroisocianuric acid, but can't remember) |
Almost certainly TCCA or NaDCCA. The red glow is given off by singlet oxygen formed from the decomposition of a peroxyhypochlorous acid species
produced in the reaction.
If the effect was quite strong and not much foam was produced, it is most likely NaDCCA. TCCA produces the same effect, but with more foaming (from my
experience) and a weaker glow.
Edit: I forgot that calcium hypochlorite produces this effect too. However, I think the effect is short-lived as compared to that from the other two
chemicals.
[Edited on 27-3-2015 by blargish]
BLaRgISH
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I'm pretty sure W. Oelen has some stuff on his site about it, maybe in the physics section.
|
|
|
badboy39560
Harmless

Posts: 2
Registered: 8-11-2014
Member Is Offline
Mood: No Mood
|
|
Please explain how to convert a sulfate into a hydrochloride?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
@Badboy39560 - It might help to be more specific. Different oxidations states of metals can make this more or less a different operation.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
For a general answer, add a hypochlorite salt to a solution of sulfate, but there are so many exceptions, that that generalization isn't useful.
What's the salt?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by The Volatile Chemist  | | For a general answer, add a hypochlorite salt to a solution of sulfate, but there are so many exceptions, that that generalization isn't useful.
What's the salt? |
He said "hydrochloride", not "hypochlorite". Methinks it is the hydrochloride of some alkaloid he's trying to make.
Deprotonate it with some base, extract the neutral alkaloid into some organic solvent, then add hydrochloric acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
No, no, see, you're suppose to give them two 'possible routes, one being the legitimate route, and one obviously not (i.e. heat with dilute
bromine-water) 
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Is camphor readily soluble in xylenes? I searched, but wasn't able to find anything on the subject. Due to its high solubility in most common organic
solvents, it seems like it would be, I'd just like to know for certain. Thanks.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
According to an old 1905 book, <i>Transactions of the Wisconsin Academy of Sciences, Arts, and Letters, Volume 15, Part 1</i> (from Google
Books), "camphor is very soluble in toluene," so I assume it would be in xylenes.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
from 36-37 question, you are correct
Quote: Originally posted by The Volatile Chemist  | Quote: Originally posted by quantumcorespacealchemyst  | What is the white frost on glass from being near HCl(aq)? It is wipable, smells odd and forms even near sealed acid containers. It seems to certainly
be from HCl gas. What it is, I don't know.
[Edited on 22-2-2015 by quantumcorespacealchemyst] |
I get it all the time on things in my lab when evaporating HCl solutions. I think it's condensed HCl, or a salt of Cl-, that is, if you
have ammonia vapors nearby or something. |
yes, you are correct.
i could not understand how it happened, not thinking about the blue solution of ammonia and copper salts i had been saving sealed in a yogurt
container with saran wrap under the lid . i just checked it and most of the water is evaporated.

thanks
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
what salt from human urine is insoluble in water and pure etanol?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
None normally, hence the reason why urine, which is water based, is transparent.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
ATX power supply question
Can I use the +12V and +3.3V outputs on an ATX power supply to make an 8.7V output? Current would flow out of the 12V output and into the 3.3V output.
(I have a Peltier cooler that I want to run that only accepts 8.3 to 8.8 volts, and draws 4 amps.) I don't want to ruin my ATX power supply by
experimentation.
Edit: More research says that my power supply should be able to handle it.
Edit 2: Testing shows that the power supply just shuts off when I try to do it (probably some protection mechanism).
Edit 3: Even more research says that it is only possible if the 3.3V output is driving something that uses more than 4 amps. (The 3.3V output cannot
sink current, only source it.) However, running the Peltier cooler off 5 volts works well enough for my purposes, I've found out.
[Edited on 7-5-2015 by Cheddite Cheese]
[Edited on 7-5-2015 by Cheddite Cheese]
[Edited on 8-5-2015 by Cheddite Cheese]
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
so now I have one and one problem! 
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
the fast, best and cheap is to buy a voltage reductor, this come in a integrated circuit like a transistor with 3 pins. perhaps not of 8,7v but 9v...
but in reality electronics have a wide range of tolerance!!! 
|
|
|
Ramium
Hazard to Others
  
Posts: 144
Registered: 3-12-2014
Location: new zealand
Member Is Offline
Mood: Licit fish
|
|
question
is there any feasible way I can convert dichloroisocyanuric acid to sodium dichloroisocyanurate?
thanks
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Before I had any idea what I was doing, I managed to make a very small amount of potassium dichlorocyanurate from TCCA and KOH. I got some of the
characteristic purple copper complex I was aiming for but extremely small amounts. It turns out this family of chemicals is a bit weird and there are
multiple side reactions and alternate reactions. The dichloro milecule was merely an intermediate on the way to something else.
So, I guess it would be possible but might be tricky. woelen explained it rather well when I asked a similar question. He did some extensive work on
these chemicals about 6-8 years ago. Good info is on the board but you will have to search. You might start by searching on me (oct last year) and see
where that takes you.
|
|
|
Ramium
Hazard to Others
  
Posts: 144
Registered: 3-12-2014
Location: new zealand
Member Is Offline
Mood: Licit fish
|
|
thanks. it sounds tricky so i'll probably just buy the sodium dichloroisocyanurate
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
europium(III) 6,6,7,7,8,8,8-heptafluoro-2,2-methyl-3,5-octanedionate
Is this the correct systematic name for EuFOD?
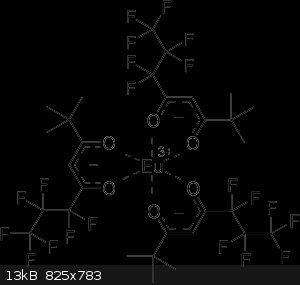
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Brain&Force - I don't think so. Ligands have special naming conventions and that doesn't appear to follow them (to me). I never cared much for
hugely esoteric molecules and the names of them though. Anyways, if you wanna get inundated, http://en.wikipedia.org/wiki/IUPAC_nomenclature_of_inorganic...
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
I reacted 2g of sulphur and 1g of aluminium - thermite reaction - and got 1.9g of slag/product. I'm wondering what happened to the 1.1g that's
missing. Maybe part of the sulphur is vaporizing instead of reacting with the aluminium?
|
|
|
diggafromdover
Hazard to Self
 
Posts: 84
Registered: 24-2-2015
Location: New Hampshire
Member Is Offline
Mood: Inconherent
|
|
Some is vaporizing. Some may be combining with oxygen. Suggestion: Figure out the stoichiometry of the reaction. Likely aluminum is the limiting
factor. Find out how many moles you started with, and how many moles of Al2S3 you would expect at 100% yield.
I would guess that any unreacted sulfur did volatilize and the slag is alumina.
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Digga!
I was assuming 2Al + 3S → Al2S3
roughly 2x27 + 3x32 ~ 54/96
so I used 1/3 Al 2/3 S - that's where the 1g/2g mix came from. Now I was thinking that if part of the S vaporizes, maybe I should use an excess of
it?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That doesn't happen, in my experience. Stoichiometric ratio is good.
|
|
|
| Pages:
1
..
37
38
39
40
41
..
104 |