Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Nitrogen dioxide mitigation in production of silver nitrate?
I am going to make some silver nitrate some time later this week from silver bullion and nitric acid. This isn't so bad to do with good ventilation
(outside) but it's the kind of thing that gets non-chemist hobbyists screaming bloody murder because it seems innocuous but can kill you dead if you don't know what you're doing and just
throw the two together. And of course that's because the reaction produces nitrogen oxides.
Mostly:
Ag + 2HNO3 --> AgNO3 + NO2 + H2O
Anyway, this isn't terrible but I'd rather avoid it. When I was looking into NOx scrubbers I ran across a lot of references to the use of
hydrogen peroxide, like this one from Solvay. NOx is scrubbed out by oxidation, hydrogen peroxide is a great oxidizer in acidic conditions.
Like so:
2NO2 + H2O2 --> 2HNO3
So I wondered if an excess of hydrogen peroxide in the nitric acid/silver reaction would convert much of the NO2 back to nitric acid before
it could escape. Of course silver decomposes hydrogen peroxide which could potentially result in some even less pretty products:
NO2 + O2 --> NO + O3
But I also found a patent related to this. (Yes, these can be exaggerated or fabricated [which scientific articles never, ever are.*cough*]) This
describes oxidizing silver with a solution of nitric acid and hydrogen peroxide (20-30% excess), both in an unspecified reactor and by pumping the
solution through a column reactor packed with silver particles. The latter was supposedly capable of eliminating NOx entirely.
I am curious to try this. I do not have anything I can use as a column reactor nor do I want to grind silver very finely so I think I will do it by
dropwise adding a solution of 20% HNO3 with ~30% excess H2O2 (relative to the nitric acid) to silver metal in a
little water.
Before I try, though, it doesn't seem likely to me, but are there any additional precautions I should be taking over the "normal" reaction of silver
with nitric acid? And is there any way I can reasonably quantify how much NOx I wind up generating? This procedure from the EPA seems like the worst nightmare.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
what if you take a rubber balloon and connect it on the exit of your vessel - it'll collect NO2 which will redissolve in nitric acid and re-react once
again.. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It seems that Silica Gel absorbs NOx and will release it when heated.
Add a column stuffed with Silica Gel to the exit of your rig.
Not sure a Balloon would hold up against NOx, although i like the idea.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
The most important question, what scale are you working on? Multi-gram scale you can always cobble something together. Multi-kilogram scale you're
going to want to invest and do things right (and also start to look at recovery to reduce waste). It's going to make a huge difference in how you
proceed.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Gram scale, I'm not interested in making profit, I just need some silver nitrate and was wondering about whether this was feasible or if anyone would
catch some potentially risky byproducts I hadn't accounted for.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Scrubbing in H2O2 or collecting with silica gel are routes to HNO3 production. See the competition thread for details. DAC#3.
You can also bubble your excess gas through a solution of NaOH which works pretty well. You don't get any useful products but it is quick and easy to
set up a bucket that will do the trick.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by papaya  | what if you take a rubber balloon and connect it on the exit of your vessel - it'll collect NO2 which will redissolve in nitric acid and re-react once
again..  |
If the nitrogen dioxide doesn't react with the rubber.
I suggest using a stoppered flask, with a tube leading into a sodium carbonate or hydroxide solution to absorb the gas.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I think Etaoin Shrdlu was talking about using hydrogen peroxide directly in the reaction mixture to not only absorb the NOx, but recycle it
by regenerating the nitric acid and thus using the hydrogen peroxide as the active oxidant in the reaction. The only problem I can see is, as he
mentioned, the silver ions may catalytically decompose the hydrogen peroxide before it has the chance to react.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by gdflp  | | I think Etaoin Shrdlu was talking about using hydrogen peroxide directly in the reaction mixture to not only absorb the NOx, but recycle it
by regenerating the nitric acid and thus using the hydrogen peroxide as the active oxidant in the reaction. |
Bingo!
Either I have a huge problem with adding too much extraneous information or people only read the title.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Apologies. I should do more than just skim read.
Well, collecting the NOx evolved using an H2O2 trap would be a first step to quantifying the amount produced. You might be able to visually assess
the amount of gas produced and get a qualitative result.
Sometimes there is only one way to find out. I would do a trial run using copper first. May as well save the expensive Ag.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I believe I tried that (actually with copper, not silver) a year or so back. I couldn't find the results in any of my lab books unfortunately, but
from what I remember it does work a little. Not as much nitrogen dioxide was observed, but still a little.
At the time I only had 6% hydrogen peroxide, so I probably used around 5 parts of that with with about a part 68% nitric acid.
Now I've got 35% peroxide but I think it will work better in a dilute solution, more time to react and more water to dissolve nitrogen dioxide.
I'd just try out if I were you. I'd do it myself, but I've already converted all my silver into nitrate or acetate!
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
i recently reacted a 1 troy ounce, 31g silver coin with 69% nitric acid to get silver nitrate.
The reaction does not start easily but when warmed the reaction starts
it is so exothermic that it heats the acid and reaction rates increase rapidly
so if warming, remove heat as soon as you see NO2.
I have a small suburban garden and the volume of NO2 from 31g of silver is easily disperesed in my small garden.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Just try baloon, either with h2o2 or without, is this too hard to try?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you can, do the reaction outside. When you work on a gram-scale, then the amount of NO2 produced quickly is diluted and taken away. No need to
scrub, just step back a few meters while the reaction is running and it is best to have wind from the back.
If you do this reaction inside, then you need a scrubber.
Also keep in mind, that this reaction also produces very fine droplets, due to the bubbling of the gas. These droplets contain small amounts of
silver(I) ions. This is something to worry about when you are inside (dark stains, the liquid evaporates, fine dust remains in the air, which can be
inhaled). Silver(I) ions are fairly toxic. If you do the reaction without scrubber, then cover the reaction vessel with a piece of paper tissue, such
that gas can escape along the paper tissue, but tiny droplets are absorbed.
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
I also recently performed this reaction outside just the other day with a troy ounce of silver. I did not use any sort of scrubber, and although the
amount of NO2 produced was substantial, it quickly dissipated in the air around. I would recommend the use of a tall form beaker to reduce
evaporation of the acid, or a round bottom flask filled with ice on top of the beaker to act as a crude reflux (if one is just carrying out the
reaction in such a vessel). I also had some minor issues with foaming due to solid silver nitrate precipitating from the solution as a result of too
much acid evaporation, although this may have just been from poor heat control on my part.
In regards to a scrubber, H2O2 is probably your best bet, since it also regenerates nitric acid. Be sure to have it in an ice
bath to increase efficiency of the absorption.
BLaRgISH
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by j_sum1  | Well, collecting the NOx evolved using an H2O2 trap would be a first step to quantifying the amount produced. You might be able to visually assess
the amount of gas produced and get a qualitative result.
Sometimes there is only one way to find out. I would do a trial run using copper first. May as well save the expensive Ag. |
I do have some atomized copper, still waffling over whether or not to jump right in though. I think I'm just going to check everything visually for
now (because I'm lazy). If there's obviously less NO2 coming off I may try to quantify it later.
Quote: Originally posted by Molecular Manipulations  | | I believe I tried that (actually with copper, not silver) a year or so back. I couldn't find the results in any of my lab books unfortunately, but
from what I remember it does work a little. Not as much nitrogen dioxide was observed, but still a little. |
Awesome! That sounds promising.
No fear of me trying it indoors until I have some practice runs down.
Quote: Originally posted by woelen  | | Also keep in mind, that this reaction also produces very fine droplets, due to the bubbling of the gas. These droplets contain small amounts of
silver(I) ions. This is something to worry about when you are inside (dark stains, the liquid evaporates, fine dust remains in the air, which can be
inhaled). Silver(I) ions are fairly toxic. If you do the reaction without scrubber, then cover the reaction vessel with a piece of paper tissue, such
that gas can escape along the paper tissue, but tiny droplets are absorbed. |
Great idea, that's something I wouldn't have thought about.
Quote: Originally posted by blargish  | | I would recommend the use of a tall form beaker to reduce evaporation of the acid, or a round bottom flask filled with ice on top of the beaker to act
as a crude reflux (if one is just carrying out the reaction in such a vessel). |
Clever. I have round-bottom flasks so I think I'll try that.
Man, you're really invested in this balloon idea, aren't you?
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I will do it myself when next time I need some copper nitrate 
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
You should. 
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Turns out I didn't have enough hydrogen peroxide to convert a troy ounce like I wanted, so it was back to the usual method. I'll try this next
chemical order.
|
|
|
ahill
Hazard to Self
 
Posts: 57
Registered: 8-1-2015
Member Is Offline
Mood: triumphant
|
|
Hey Etaoin,
I made silver nitrate the other week - I was very happy with the end result - but I ran into quite a few issues along the way.
Some of the contraints I have are :-
I dont have awesome ventilation - an airy garage , but no fume hood.
The backyard is kind of public
I've got little kids
The reaction can (and did for me) take quite a while to get going
The product is light sensitive
..and of course - it produces lots of corrosive poison gas, over a significant period.
In the end, I set up a gas bubbling apparatus using a couple of beakers and a glass dish containing a strong NaOH/water solution like in the attached
image. Gas still escaped - but the volume and noxiousness was much reduced - and I was able to let the reaction proceed in a gloomy and safe spot in
my garage.
The final product was beautiful clear crystals which I dryed using a similar(ish) construct using dry NaOH instead, and covered with clingwrap.
..oh yeah - and watch for suck-back - when everything is done - the NaOH will suck back into the big beaker - and not gently - it will GLUB - causing
quite scary splashing which will contaminate the contents of the little beaker if it isnt big enough.
Let us know how you go.
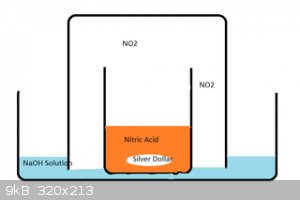
[Edited on 26-3-2015 by ahill]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ahill  | Hey Etaoin,
I made silver nitrate the other week - I was very happy with the end result - but I ran into quite a few issues along the way.
Some of the contraints I have are :-
I dont have awesome ventilation - an airy garage , but no fume hood.
The backyard is kind of public
I've got little kids
The reaction can (and did for me) take quite a while to get going
The product is light sensitive
..and of course - it produces lots of corrosive poison gas, over a significant period....
[Edited on 26-3-2015 by ahill] |
For those interested in small quantities, there may be an effective and safer path to Silver nitrate. While the most known route appears to be the
direct action of HNO3 on Silver metal, there are, in fact, others. To quote, for example, from one source, Atomistry.com on AgNO3 (link: http://silver.atomistry.com/silver_nitrate.html ) on the preparation of Silver nitrate:
"Silver, Silver monoxide, Silver sulphide, and Silver carbonate dissolve in nitric acid. Concentration of the solutions yields colourless, rhombic
crystals of Silver nitrate, AgNO3, of melting-point 208.6° C."
Also, for those interested in preparing other salts of Silver or as a precursor to Ag2O, like for example, Silver citrate, see Page 4, Equation (3) at
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2590638/ further illustrating the use of Ag2O in the preparation of Silver salts.
----------------------------
Interestingly, I have fairly quickly prepare Silver acetate (in a few hours) by adding Silver metal to dilute H2O2, vinegar and some KNO3 to serve as
an electrolyte (and not my normal recommendation of sea salt, as a chloride will not work here) for the electrochemical cell. I briefly heated in a
microwave to jump start the reaction, and added more H2O2 and/or vinegar as needed to keep it going. The first picture below was actually taken within
the 1st hour and shows that one can prepare (cheaply and safely) a Silver salt from the metal in a matter of hours in limited quantities.
Note, since KNO3 is employed here, I should mention that it is not a completely passive electrolyte. In fact, with light, both H2O2 and KNO3 can form
hydroxyl radicals as I have detailed previously (see, for example, http://www.sciencemadness.org/talk/viewthread.php?tid=34429#... ). So with time, the solution forms a black precipitate as the acetate ion is
attacked and eventually, in the presence of H2O2 also, I suspect leads to the formation of the black Ag2O. On further boiling with Na2CO3, a clear
solution (containing aqueous NaOH) develops with a tan precipitate of Ag2CO3 (see last picture below).
-----------------------------
It is also interesting to note the following property of which is shared at least with Silver acetate:
"Although, silver citrate is minimally soluble in water, it can successfully be dissolved in citric acid solutions. The maximum concentration of Ag(I)
in solution is estimated at 23 to 25 g/L if the concentration of citric acid is at least 4 mol/L or higher. The dissolution of silver citrate in
citric acid solutions was attributed to the formation of silver citrate complexes of a general formula [Ag3(C6H5O7)n+1]3n−. "
So, one may be able to successfully apply any parent acid (including HNO3) to Ag2O (and also consider Ag2CO3) as routes to Silver salts including
AgNO3


[Edited on 5-4-2015 by AJKOER]
|
|
|