| Pages:
1
2
3
4 |
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Quote: Originally posted by Oscilllator  | For what it's worth, I use density measurements almost exclusively when trying to determine the concentration of acids and other substances. Sulfuric
acid is ideal for a density measurement, because the density of pure sulfuric acid is about 1.8.
A 100ml erlenmeyer flask and a decent scale will tell you the concentration of your acid to within a few %, and you don't have to mess around with
titrations and other such shenanigans. |
Oh wow! I can't believe I hadn't thought of that. That sounds like a fantastic plan of action, and I think you already have some 0.01g scales don't
you Trizocy? A grad cylinder or a burette would be better than an erlenmeyer flask though I would think.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TheAustralianScientist  | Quote: Originally posted by Oscilllator  | For what it's worth, I use density measurements almost exclusively when trying to determine the concentration of acids and other substances. Sulfuric
acid is ideal for a density measurement, because the density of pure sulfuric acid is about 1.8.
A 100ml erlenmeyer flask and a decent scale will tell you the concentration of your acid to within a few %, and you don't have to mess around with
titrations and other such shenanigans. |
Oh wow! I can't believe I hadn't thought of that. That sounds like a fantastic plan of action, and I think you already have some 0.01g scales don't
you Trizocy? A grad cylinder or a burette would be better than an erlenmeyer flask though I would think. |
Damn now we are back on density again, it was this the previous forum also recommended me to do :/
Yes i got 0,01g and have aldready done some measurement with density, and here is the result:
I got a 1L bottle of sulfuric acid, on the bottle it stand 60-100%, when i measure it with 50 mL i got 93,34g
93,34g * 2 = 186,68g
186,68g / 100 = 1,8668 g/ml density
And here is the density table for it: http://www.sschemical.com/wp-content/uploads/2013/05/Convers...
The highest % here is 1,8391 g/ml, but my result is 1,8668???
Also they recommoned me to buy a pycnometer, which is the only way to be truly accurate it they said.
With only burette,pippette or a cyilnder i could never pour on the same amount like last time.
Had a expriment with density and freshwater
Quote: Originally posted by me  | | I had a brand new fresh 5L water (think its filter water, and yeah! i know that distilled water and filter water is not same, but this was just a
experiment on it, gonna try the destilled water when i got some) so i tryed to measure it 20 times, and here was the result: http://pastebin.com/2xSHGNkq - it comes between 48,14g and 48,95g on 50 ml (20-21°C), (average 48,5905g on 50 mL) My standard deviation was
0.27458. |
As you see in the link, almost never got the same number.
Thanks
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Trizocy, you got higher density due to some error - rather measuring at different temperature or most likely error in volume measurement. But you know
what - if you can't take exact volume of acid, how do you expect titration to work for you?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Trizocy-
If the density of water doesn't work out to be 1g/cc with your equipment, either your equipment or measuring technique needs looking at.
For a start: When using the graduate cylinder, you will observe the "meniscus", a curvature of the liquid's surface due to surface tension. How are
you accounting for that?
http://en.m.wikipedia.org/wiki/Meniscus
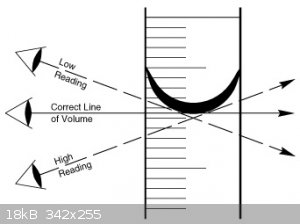
[Edited on 19-2-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Hmm i think its my measuring technique which must be wrong 
Anyway, if i gonna measure density, then i could just buy pycnometer.
Pycnometer (25ml) on 0,01g scale. Note down the Pycnometer weight
Pour Sulf acid in the Pycnometer and put the top on, let acid overflow on the pycnometer.
Put back on scale, to measure the mass
Pycnometer weight - Pycnometer full of sulf acid = mass of sulf acid
Volume / Mass = Density  ? ?
Then you find what tempture it is, and note that down. And so find specific gravity density sulfuric acid. And lookup where you result is = Profit
 ? ?
But one problem, i havent found a legit confirm authority specific gravity, there are too many of them on google, and all have difference measuring :S
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Density measurements with Pycnometers are usaually done relative to pure water. So also measure Pycnometer weight when filled with water.
W0 = pycnometer weight
W1 = pycnometer filled with acid weight
W2 = pycnometer filled with water weight
Density relative to water = (W1 - W0)/(W2 - W0)
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Hello!
Let's finish this thread 
So... Lets continue.
I got a pycnometer now, so i can try to measure the density now
I got 1 Liter of H2SO4, which on the description it stand 60-100% and what i want is to try to find the specific concentration in the bottle.
So what i have done is to measure several times with a 50ml pycnometer, and a temperature meter.
50ml pyconmeter empty: 34,15g
Average 50ml pycnometer with H2SO4: 126,91g (pour in and out several times, and measure it)
Weight of the H2SO4: 92,76g
Temprature: 7,5 cel to 10,5 cel
So how do i calculate it? Tryed to use Chembuddys calculator, but gave me 185% concentration which is not possible 
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
You know both the mass AND the volume of this mass of liquid, don't you see it? Just think about the unit's you have and what you're looking for...
92,76g / 50ml = 1,855g/ml. Then it's just a matter of looking this up in a table.
BTW: Well done, a pycnometer is the proper tool for this. I've always had trouble with getting good measurements from hydrometers.
[Edited on 19-3-15 by Fulmen]
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
It seems to me that you have some VERY concentrated acid, although I think there may be a little error in the calculation.
Azeotropic (98%) Sulphuric Acid has a density of 1.84g/mL so I would say you have overshot a little, but even so, going off that density reading I
would say you have at the very least 97%.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Small amount of dissolved SO3 would throw this off wouldn't it?
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Hello guys!
Fulmen:
I aldready know that, but here is the problem, my calculation ending up at 1,855g/ml but the highest concentration of H2SO4 is 1,84g/ml so something
is wrong here.
TheAustralianScientist:
How can i do a overshoot when i got a pycnometer?  ? ?
Hyfalcon:
May u deepen more about u talking about?
Thanks all!
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Trizocy,
Are the weighing scales calibrated ?
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Nope 
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Remember the pycnometer is only instrument 1 of 2 in the density measuring process. The scales are equally important, so if the scales are off, your
calculation of density will also be off.
Calibration weights are fairly cheap, you just need to get the one that your scale requires.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Also density data are given for specific temperature and your picnometer volume depends on temperature as well, also impurities in sulfuric acid can
affect density, since 1.84 is given for azeo H2SO4, which must contain only water.. Better to titrate it, but here you'll come to primary standart
preparation problem, yes analytic work is hard to do at home.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Start by testing a few known chemicals like water, ethanol etc, that will give you an idea of the accuracy.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
| Pages:
1
2
3
4 |