| Pages:
1
2
3 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Hydroxylamine NH2OH Uses (and Production)
Well I have a batch of hydroxylamine sulphate lying about - about 500 g, from a photographys supplier.
I wondered what kind of experiments I could perform with it?
This is what I came up with:
It reacts with ketones/aldehydes to for the respective oximes, i.e.
acetone + NH2OH --> (CH3)2C=NOH
which can be crystallised out in cold water.
Then, one can make nitrous oxide, N2O with it 
2HONH2 + 4CuO = 2Cu2O + N2O + 3H2O
Very neat method I think, one gets valuable Cu2O and N2O, too!!
Also, there is another one:
R-COOR' + H-NHOH = R'OH + R CO'NH'OH
Meaning that any carboxylic acid ester such as ethyl acetate will react with it, forming a hydroxamic acid.
Also, this produces coloured complexes with Fe3+.
I think there are many more interesting reactions, if you are aware of any please post away!!
[Edited on 22-2-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
I expect hydroxylamine nitrate would be fairly interesting. 
[Edited on 28-4-2004 by I am a fish]
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
You can use for a reductive amination - phenylacetylcarbinol to phenylpropanolamine via the oxime.
This forms an interesting oxazoline compound when reacted with KOCN as I was told......
Phenylacetylcarbinol is made by bakers yeast/benzaldehyde - should be not to hard for ya I guess.
(Neuberg/Hirsch 1921)
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
What exactly is phenylacetylcarbinol?
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
CAS 90-63-1 (1-hydroxy-1-phenyl-2-propanone)
Formula: C9H10 O2
Composition: C, 71.98; H, 6.71; O, 21.31
Mol. Weight: 150.1745
Exact Mass: 150.06808
1-hydroxy-1-phenylacetone, aka Acetylphenylcarbinol, boils at 205-7° C at atmospheric pressure, 135-7° C at 24 mm Hg, 140-5° C at 11 mm Hg and 66°
C at .2 mm Hg It's miscible with most organic solvents.
C6H5CHOHCHOCH3
1-hydroxy-1-phenyl-2-propanone
1-hydroxy-1-phenylpropanone
1-phenylpropan-1-ol-2-on (phenylpropanolon) -> old german
phenylpropanolone
1-phenyl-2-ketoalcohol-(1)
1-phenyl-propanol-1-one-2
PAC
Acetylphenylcarbinol
phenylacetyl carbinol
1-phenylpropan-1-ol-2-one
1-hydroxy-1-phenylacetone
1-phenyl-1-hydroxyacetone
Benzacetoin (german)
Funny:
The mostly referred form is laevo-phenylacetylcarbinol aka
l-phenylacetylcarbinol aka
l-PAC aka
L-PAC aka
R-PAC (thats a good one hey!)
because of:
(R)1-hydroxy-1-phenyl-2-propanone is correct for laevo-phenylacetylcarbinol.
Even more funny is that phenylacetylcarbinol is WRONG as this describes an isomer - but as Neuberg and Hirsch who discovered the stuff made this
mistake in their first publications (corrected it lateron) the name is still used.
Do you now know why I HATE chemists?

|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well of course, the hydroxylamine nitrate/perchlorate are things to try - they have a large oxygen excess, so I wouldn't try it without something
combustible.
Anyway, I seem to recall that there are some reactions on the phenylgroup, with hydroxylamine. Does anyone know more?
Also, what can one do with oximes?
Reductive hydrogenation, to what? Amines?
Kaboom, we need your input ! 
PS Org, so what is the phenylcarbinol used for?
[Edited on 28-4-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Hjalmar_Poelzig
Harmless

Posts: 20
Registered: 4-8-2003
Member Is Offline
Mood: No Mood
|
|
phenylacetyl carbinol
phenylacetyl carbinol is a precursor for both norephedrine (aka phenylpropanolamine) and ephedrine.
For the first the oxime is made using NH2OH, followed by reduction to the amine.
For the second the ketimine made in situ from methylamine and PAC is reduced to the subst. methylamine.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I just had this thought - H2NOH forms oximes with aldehydes and ketones, as we all know.
However, what if we mix hydroxylamine and formaldehyde?
We will get H2C=NOH, which presumably will polymerise in a certain fashion. Now the question is, it will polymerise to what?
In the analogous sitation, ammonia NH3 and formaldehyde CH2O produce hexamethylenetetraamine, which we all know, and it's applications
thereof (hint hint  ) - so what will happen with hydroxylamine? ) - so what will happen with hydroxylamine?
Lets bring out the organic chemists!!!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Turel
Hazard to Others
  
Posts: 141
Registered: 29-11-2003
Location: The Hardware/Software Interface
Member Is Offline
Mood: Thixotropic
|
|
Organic Chemistry
Organikum: That oxazoline is indeed interesting, as I'm sure you know. I've been interested in 4-MAR for a few years.
madscientist chemoleo: levo-phenylacetylcarbinol (L-PAC) is the biochemical precursor to PPA and ephedrine, from which plants produce these two
compounds in nature. Microorganism exploitation leads to the same result in a petri dish or larger reactor.
Methylene hydroxylimine could polymerize in two different ways. It could repeating chains of [-CH2N(OH)-]n where the methylene carbon and nitrogen
form the linkages. In my opinion this rather low stability bonding would likely form small rings able to be stbilized by resonance/tautomerization of
the hydroxyl to a nitroso function.
The other method would form longer chains of compounds of repeating [-CH2N(H)O-]n functions where the methylene carbon forms bond with oxygen and
nitrogen. This will also be prone to hydrolysis as the first is.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Maybe reacting formic acid with hydroxylamine will give you fulminic acid? 
NH2OH + HCOOH => HCOHNOH
HCOHNOH => HNCO + H2O
Edit: Hydroxylamine, I think, would react with formaldehyde to from a hexamethylenetetramine type compound, except that every nitrogen will have a
coordination covalent bond (donation) to an oxygen atom. From this compound, HMX or RDX could be made which has either a nitrate group attached to the
nitrogen atoms of the ring (instead of a nitro group), or every ring nitrogen atom will have an oxygen attached to it. This tri-N-oxo RDX or
tetra-N-oxo HMX will have perfect OB and will presumably be more powerful.
[Edited on 21-10-2004 by Theoretic]
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
You could make sodium or silver hyponitrite (Ag2O2N2). The prep in Am. Chem. Soc. Oct. 1924, pps. <2175 involves the use of hydroxyl amine as one
of the starting agents for this compound. I only have a paragraph of the article photocopied (the last paragraph, copied it when I was copying the
article following it, and whenever I saw that last paragraph I thought of this thread.).
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Does anyone have a synthesis for hydroxylamine? I searched the net, and all I found directly relating to hydroxylamine synthesis was this http://www.korozja.pl/131_01_03.pdf . Not that you need to synthesize it, it's pretty cheap at around $25-50 per kilogram, but it would be
good to know.
Edit: Nevermind, I found this from Rhodium:
Preparation of Hydroxylamine hydrochloride
40g potassium nitrite (KNO2) and 50g potassium acetate (CH3COOK) are dissolved in 100ml ice water. 750g finely crushed ice is added. Into this
solution a stream of sulfur dioxide (SO2) is bubbled until the solution smells of SO2. The temperature must be kept below 0°C through the whole
reaction. The salt of K2[HON(SO3)2] separates and is filtered off and washed with ice water. The salt is dissolved in 500ml 0.5 M HCl and boiled for
two hours. Still boiling a solution of Barium chloride (BaCl2) is added as long as barium sulfate (BaSO4) precipitates (maybe CaCl2 will do instead).
The BaSO4 is filtered off and the clear filtrate is evaporated to dryness. The residue consists of Potassium Chloride (KCl) and NH2OH*HCl. Anhydrous
ethanol(EtOH) is used for extraction of the Hydroxylammonium chloride, the KCl remains undissolved. The EtOH is evaporated on a water bath and the
product can be recrystallised from water (mp 151°C).
[Edited on 27-11-2004 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
From:
MICROWAVE SPECTRUM OF FORMALDOXIME
M. G. Krishna Pillai;
J. Phys. Chem.; 1962; 66(1); 179-180.
| Quote: | | "Formaldoxime was prepared by mixing an aqueous solution of formaldehyde with a solution of hydroxylamine hydrochloride to which sufficent
sodium carbonate had been added to liberate the hydroxylamine(2). From the mixture formaldoxime was extracted with ether. The polymer of formaldoxime,
obtained by the evaporation of the etheral solution, gives our the monomer when heated." |
(2) W.R. Dustan and A.L. Bossi, J. Chem. Soc., 73, 353 (1898)
Picture of formaldoxime attached 
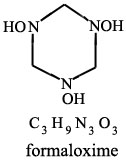
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Excuse my lack of ochem knowledge, but could this be mixed with nitric acid to form RDX? Maybe it could be nitrated into some other interesting
explosive?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Formaldoxime and potential nitrates?
Of course, that's the whole idea. I recently tried this experiment, and the solution of hydroxylamine and CH2O heats up considerably. Until
recently I didn't know the products, thanks to Bromic and myself for having the ingenious idea that the product might be called
'formalDoxime' (rather than formaloxime which yielded no search hits)
Anyway, theoretically there should be a trinitrate, practially probably only a dinitrate exists (or diperchlorate  ) )
This is something that ought to be tested. It is likely to be more powerful than hexamethylene tetramine dinitrate (due to a distinctly better OB)
However, I don't think that an analogue to hexogen/RDX can be formed, because there is no free hydrogen attached to the nitrogen. I.e.
hexogen/RDX is a nitramine, i.e. a nitro-amine, which is not possible in the case of formaldoxime.
[Edited on 22-2-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Caffinehog
Harmless

Posts: 9
Registered: 3-3-2005
Location: Under a rock somewhere
Member Is Offline
Mood: Unstable
|
|
Uses of hydroxylamine..... How about converting ketones to amides?
Mix it with a ketone. This forms the oxime. Then add a medium strength acid, like phosphoric acid, and warm it. This causes the Beckman
rearrangement. You get an amide, with primarily the larger organic group on the nitrogen. Acetone ----> N-methyl acetamide. Acetophenone
---->acetanilide.
Heat it too much or/and add to strong an acid, and you will hydrolyze it to the corresponding carboxylic acid and amine. For example, acetophenone +
hydroxylamine + heat +HCl ----> acetic acid + aniline
I don\'t understand what you are saying, but I agree with every word of it!
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
It won't be a nitramine, this HMX analog, but it will be an oxamine, since the acid would react with the hydrogen atom of the hydroxyl group
attached to the nitrogen to leave the nitro group in its place. A whole new class of explosives, oxamines! 
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Caffinehog, that's very interesting. An easy route to amides - with OTC hydroxylamine - nice. Here's some more detail on the Beckmann rearrangement. Although they actually mention conc H2SO4. I guess it depends on the type of oxime.
I was wondering, can the oxime be reduced easily to the respective amine? I imagine this needs rather strong reducing agents, such as LiAlH4 etc. Or
electrolysis?
I.e. according to
CH3COCH3 + NH2OH --> CH3C(=NOH)CH3 --> CH3HC(NH2)CH3 (2 propylamine)?
Theoretic, as far as I know formaldoxim is hydrolysed back to hydroxylamine and CH2O in the presence of acids. So this wouldn't work.
Are you talking of a NITRATE group on a nitrogen? I.e. R2N-O-NO2? I don't even know whether that exists.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
If you use a direct nitrating agent (a compound of the NO2+ ion) like NO2HSO4, hydrolysis could be avoided, not so if you use nitrating acid
(H2SO4/HNO3). NO2HSO4 can be made from concentrated HNO3 and sulfur trioxide, obtainable by decomposing ferric sulfate.
And yes, I'm talking about R2N-O-NO2. What about a nitrate group on a nitrogen? N2O3 and N2O5 do exist, and they have it.
|
|
|
Axt
National Hazard
   
Posts: 795
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I'm attaching an article which supposedly gives the synthesis of NH2OH.HCl by refluxing HCl with nitromethane.
<a href="http://xfiles.ft100.net/images/nm-hcl-hydroxylamine.pdf">Journal f. prak. Chemie, Vol 21, 1880, page 129</a>
Can a german speaker please look at that and give the specifics of the reaction (quantities, reflux time, extraction etc.). If its in there ...
somewhere.
More random NH2OH articles I have:
http://scibooks.ft100.net/images/hydroxylamine.zip
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Funny that you bring this up, as just 48 hours ago I was reading this:
Attachment: iec_31_118_1939.pdf (433kB)
This file has been downloaded 1816 times
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Interesting references. I always love these old gems, with obscure structures, where things such as the structure of hydroxylamine weren't entirely
elucidated, or at least where there's some debate. Still a frontier of science of sorts.
Anyway, here's the translation for the
Preparation of hydroxylamine from nitromethane:
25-28 gram of nitromethane was heated with twice the volume of HCl (presumably 37% although this isn't specified) in a glass (test?) tube that was
melted and sealed (similar to an ampoule), and heated to 100 deg C. After cooling the tube (no mention is made as to how long, but see the below),
where higher pressure only occurred intermittendly (in other words, the glass container was able to resist the probably weak pressure), hydroxylamine
hydrochloride crystallised as glorious, almost inch-long crystals (where the remaining solution presumably consists of HCl, formic acid and H2O).
Remaining free HCl was removed by heating them for a prolonged time on a waterbatch, and by a single recrystallisation step. Yield is 18-20 g, and
compared to the Loss's procedure, it is faster and yields a cleaner product. It makes no mention of refluxing this mix - might be difficult with the
HCl present, but I don't know.
Sounds like a good method, the only drawback being a pressure container. But by the sound of it, a glass ampule is enough, so a strongly stoppered
pyrex flask in a 100 deg C bath should be fine, right?
Also, it mentions that the hydroxylamine HCl can be reacted with cyanamide to form oxyguanidin hydrochloride, CN3H5O:HCl. What precisely is this?
Anyone knows?
Cyanamide sounds like an extremely versatile compound....!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I tried something out today: In a thread on production of N2O, it was mentioned that extremely pure N2O is obtained by combining solutions of a
hydroxylamine salt and a nitrite.
It's only stupid that all sources of hydroxylamine salts are really expensive, at least those that I know. I purchased 25g of the sulfate for some
experiments.
I added 1,6g of di-hydroxylamine sulfate (0,01 mol) and 1,4g NaNO2 (0,02 mol) (stochiometric amounts, you see) to a test tube, and added 15ml water.
The salts dissolved upon swirling, and gas production started immediately, despite the solution being very cold from endothermic dissolution of the
NaNO2.
This was a marked difference to the reaction of an ammonium salt with NaNO2, which takes place only on warming.
The solution continued fizzing, and the gas evolution became stronger and the solution heated up, and as soon as it was mildly warm to the touch,
runaway occured and the solution frothed over, it went so fast that I couldn't do anything against it.
So this method produces N2O in good amounts, but temperature control is critical to prevent runaway and only dilute solutions should be used.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have tried the HCl + nitromethane reaction in an effort to prepare energetic furazan compounds ( diamino furazan can be made from condensation of
glyoxal and hydroxylamine to diaminoglyoxime followed by ring closure with aqueous KOH ).
I refluxed 20 ml NM with ca. 25 ml HCl, 30 % for about 24 hours total ( I let it cool down in the evening as I don't like heating something overnight
). The HCl was ancient ( 10-20 years ) and had a strong yellow coloration, probably from the green PE bottle which it was stored in. Towards the end
of the process, I noticed that the mix still separated into two phases ( which it obviously shouldn't if the reaction had gone to conclusion ), so I
added a little more acid and in the course of a few hours, it became homogenous.
I then cooled it down, but no crystals appeared. So, I left it sitting there and didn't get around to evaporating a sample for some weeks.
When I did get around to it, I got a reasonable amount of crystals which where soluble in ethanol, strongly reducing and quite hygroscopic. The only
crystalline impurity that I can think of is NH4Cl which is almost insoluble in ethanol ( 0.6 g/100 ml at room temp ).
Apart from the long heating period, the only problem seems to be the isolation as hydroxylammonium chloride starts to decompose around 120 C so
evaporation to dryness should be done very carefully. I ended up heating it gently until only some 10-20 ml remained, then putting it in a desiccator
over a mix of CaCl2 and CaOH.
I contemplated a redox titration to determine the purity, but this is complicated by multiple oxidation products ( N2, N2O, NO, NO2, NO3- ). I tried
acidified KMnO4 which I like because it goes all the way to colorless Mn2+. The hydroxylamine turned the KMnO4 soln completely clear without visible
gas evolution, so maybe it is safe to assume ( for a rough estimate of purity ) that the hydroxylamine is oxidized all the way to nitrate...
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I had a look in Hollemann- Wiberg on hydroxylamine, and guess what, the reaction of aqueous sulfuric acid and nitromethane is an industrial method for
the production of hydroxylamine sulfate.
Apparently, other nitroalkanes can be used as well, they get converted to the corresponding carboxlic acids and hydroxylamine.
|
|
|
| Pages:
1
2
3 |