zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
how to make this ester
My substrate is
[img]http://ccebbs.com/forum/showimg.asp?id=9323[/img]
I want to make a phosphoric ester at only the 3-OH.
How can i do it?
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
the picture cann't be seen correctly here.
you can see it in this website
http://ccebbs.com/forum/dispq.asp?lid=9323
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Oh no I can't.
|
|
|
chloric1
International Hazard
    
Posts: 1146
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
That kind of claritiy can only compare to the clarity one experiences after a long night of binge drinking. LMAO!
Fellow molecular manipulator
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Since I can't read Chinese I can only assume you mean gallic acid or methane/carbon tetrachloride which appears in the banner. Since neither are
esters you probably mean something in the text of the page, a text which I cannot read, I'm afraid that not many people here speak Chinese, if
you want a better response please provide a formula.
[Edited on 2/19/2005 by BromicAcid]
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
i think the refernce to the page is for the molecule picture....
Its 3,4,5-trihydroxycyclohex-1-enecarboxylic acid.
So he is refering to the OH group on position 3... making the phosphoric ester at this position...
Ok, now thats sorted i must say i dont know how you would do this.... but now that the whole substrate and chinese text is sorted someone else may be
able to help with the ester formation.
-rlr
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"That kind of claritiy can only compare to the clarity one experiences after a long night of binge drinking. LMAO! "
At 3 in the afternoon? OK, it was Saturday so I had been out drinking the night before, but I can usually manage to muster that degree of clarity
anyway.
(For what it's worth, when I looked at that page it was incomprehensible, and not even recognisable as Chinese, nor did the structures show up.)
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
thank everyone.
the reference is only the picture.
I'm a chinese. I'm very glad to meet with everyone in the forum.
Welcome to China.
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
runlabrun is correct.
I will try my best to draw a new picture in a new post.
thank everyone
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
how to make this ester in moderate yield
how to make this ester in moderate?
see the picture in the attachment
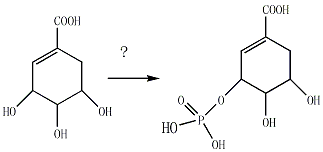
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Proposed synthesis
I'm not sure about the correctness of this, but it looks good to me.
Substrate --(CH<sub>3</sub> <sub>2</sub>CO +
H<sup>+</sup>--> acetal <sub>2</sub>CO +
H<sup>+</sup>--> acetal
acetal --P<sub>2</sub>O<sub>5</sub>--> phosphate
phosphate --H<sub>3</sub>O<sup>+</sup>--> desired product
I used non-OTC methods since I don't think the substrate would be OTC anyway. 
(edit: the only side product I can see forming is the isomer phosphorylated at the -OH opposite the double bond.)
sparky (^_^)
[Edited on 21-2-2005 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
Thank sparkgap.
The method you proposed is good.
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
| Quote: | Originally posted by sparkgap
Substrate --(CH<sub>3</sub> <sub>2</sub>CO +
H<sup>+</sup>--> acetal <sub>2</sub>CO +
H<sup>+</sup>--> acetal
|
Why wouldn´t the 3-OH react with the acetone to form mixed acetals?
Another approach would be to oxidize selectively the 3-OH.
Several reagents accomplish this task, I'll give a couple of examples:
-K2MnO4 under fase-transfer conditions oxidizes allylic but doesn't touch primary or secondary alcohols. Ref: Tetrahedron Letters, 30, 19 , 1989.
-NaNO2 and Ac2O oxidizes primary, allylic and benzylic but not secondary alcohols. http://pubs.rsc.org/ej/P1/2000/B001215G.pdf
Then react with (CH<sub>3</sub> <sub>2</sub>C(OCH<sub>3</sub> <sub>2</sub>C(OCH<sub>3</sub> <sub>2</sub> to
form the acetal, reduce to the alcohol and phosphorylate. <sub>2</sub> to
form the acetal, reduce to the alcohol and phosphorylate.
[Edited on 23-2-2005 by alchemie]
[Edited on 23-2-2005 by alchemie]
[Edited on 23-2-2005 by alchemie]
[Edited on 23-2-2005 by alchemie]
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
see also scheme 2 in this page:
http://www.albmolecular.com/features/tekreps/vol04/no48/
They selectively protect a diol as an acetonide in the presence of an acrolein (wich you would obtain from oxidation of an allylic alcohol).
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
My reasoning in assuming no (or more precisely, very little) mixed acetals would form was because of entropy considerations.
But I must admit your approach is more elegant. 
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
sparkgap:
The way I suggested is pretty much more complicated than your's.
If the two secondary alcohols form the acetal preferentialy then your's is much better. I tried to look up something about alcohol reactivity
towards acetal formation but didn´t find anything.
Another problem in both approaches is the carboxylic acid. Maybe it stays there and just watches , or maybe it has to be protected as well. , or maybe it has to be protected as well.
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
thank alchemie,sparkgap
you shed light on my question...
I learn much from your posts.
Do you have some writings about learning organic chemistry?
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
zbde00:
Very much welcome. 
Best way to learn organic chemistry? The only advice I can give is to read books on it. McMurry and Carey are pretty good in explaining the basics. If
you deem yourself ready to tackle advanced organic chemistry, you might want to check out March.
About tackling organic synthesis, Warren writes good. I can't for the life of me comprehend Corey. 
Oh, and yes, practice makes near-perfect. 
alchemie:
I gave my superior this question the other day. She told me there is no use hoping for chemoselectivity since they most likely have equal
reactivities. Thus, forming the acetal results in two possible products, one to be phosphorylated subsequently, and the other to be separated out.
She also said the carboxylic acid is not very likely to interfere. 
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|