amateur scientist
Harmless

Posts: 2
Registered: 3-12-2014
Member Is Offline
Mood: No Mood
|
|
what happened to methoxyhydroquinone?
Methoxyhydroquinone in artificial surface water decomposed into something in presence of light. The solution is now pink/grayish pink. Any idea on new
product/products?
|
|
|
Keftedes89
Harmless

Posts: 12
Registered: 3-12-2014
Member Is Offline
Mood: No Mood
|
|
Hydroquinone undergoes oxidation to benzoquinone under mild conditions. Perhaps the same is happening?
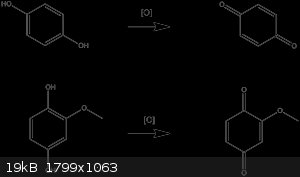
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
You might try performing an extraction of your aqueous solutions with a non-polar or weakly polar aprotic solvents to isolate the product (hexane, pet
ether, DCM, chloroform, ethyl acetate, etc). Quinones usually dissolve to varying extents in these.
From my own experience, DCM works best for 2,5-dimethoxy-1,4-benzoquinone, a methoxyquinone that is very poorly soluble in many other solvents.
|
|
|
amateur scientist
Harmless

Posts: 2
Registered: 3-12-2014
Member Is Offline
Mood: No Mood
|
|
I've been performing GC analysis to my solution(via extraction to DCM). Apparently, the concentration of methoxyhydroquinone decreased to 25%of the
original concentration in 4 days. However, this pink color (whatever the compound is) did not pass to DCM, prefered to stay in aqueous solution.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I've observed a similar thing occurring with aqueous hydroquinone solutions... surely there is literature on this considering hydroquinone's
widespread use in photography?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
The quinones (and the products of the polymerizing with the free phenol) are frequently pink. In the presence of trace amounts of iron (III makes the
colored complexes and II engages a REDOX cycle), they are frequently green, bluish, purple--added together, this can appear to be gray (at least so
far as I've seen).
So, keep them free of iron, out of the light, and preferably, away from air.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Nicodem
|
Thread Moved
3-12-2014 at 08:14 |