JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
Biochemical pathways needed to produce penicillin !
I'm confused about the large scale downstream process of Penicillin. AFAIK; it's made biochemical in a Fed-Batch reactor with D-valine (other sources
claim L-valine) + alpha-aminoadipic acid + L-cysteine in Penicillium chrysogenum.
6-APA (6-aminopenicillanic acid) is then formed in the cell walls of this fungus. That's science.
I'm not understanding a few things:
1) Is 6-APA first produced and later on a enzymatic reaction with alpha-aminoadipic acid takes place but IF this is true what happens then with the
penicillin?
What is the biochemical pathway in Penicillium chrysogenum step by step ?
I couldn't find graphs about the pO2, CO2, temperature in the bioreactor itself. Only statistical approaches that I couldn' read
like:
Wang, 1981: I don't have a credit card for a simple casus as a student, I can't pay it so...
Another (better) reference but with much statistics is this one:
Guerreiro et al (1997)
Can someone explain me how penicillin is produced ?
Then I did read the following text: in the industry they use enztmatic production to obtain 6-APA and Penicillin amidase (extracted from E.coli) is
used.
Are they talking about cephalosporins or N-Acylation of 6-APA ?
[Edited on 20-11-2014 by JAVA]
[Edited on 21-11-2014 by JAVA]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Hopefully this will help?
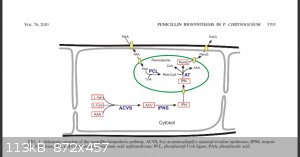
It was taken from the following paper which you should be able to get free
Meijer, Wiebe H. et al. “Peroxisomes Are Required for Efficient Penicillin Biosynthesis in Penicillium Chrysogenum .” Applied and Environmental
Microbiology 76.17 (2010): 5702–5709.
The other paper that might help you and is also free (or should be)
Veiga, Tânia et al. “Resolving Phenylalanine Metabolism Sheds Light on Natural Synthesis of Penicillin G in Penicillium Chrysogenum.” Eukaryotic
Cell 11.2 (2012): 238–249.
[Edited on 24-11-2014 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
ACVS is a D-valine transferase while the precursor is L-Valine. Don't understand that, anyone ?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JAVA  | | ACVS is a D-valine transferase while the precursor is L-Valine. Don't understand that, anyone ? |
See PMID: 11851475
Chem Rev. 1997 Nov 10;97(7):2631-2650
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Have a look at the papers I referenced I cant upload the 1.6meg file (my internet), also look at the paper chemosynthesis gave you, it give a better
chemical perspective than the biological one I posted.
If your trying to grow small reactor amounts then consider this.............. It was grown on window sill then isolated a very long time ago. Standard
Trip plates and ~18-27C is about right, CO2 minimal in a small system.
If you want industrial scale then forget the papers I linked too, In industrial reactors the pathways are non standard and the reactor is normally
anaerobic, you first need to decide your scale and pathway,the above pic is the standard pathway for say if you had a petri dish sitting on your bench
top, however under anaerobic conditions the pathway would change, you also need to consider how to eliminate the unwanted microbial growth you would
get.
I am not sure what your after as you asked about temp and CO2 etc, so are you just looking into how its done in industry or are you wanting to
replicate it and make penicillin? It kind of makes a difference regarding papers I would link too.
Dont ask me, I only know enough to be dangerous
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
It's D-valine instead. 
But the parameters are now known for P. chrysogenum because I have to wait too long for get a scientific based answer in this section and
lack time:
pH 6,5-7,7 (phosphate buffer)
oxygen supply: 25-60 mMol/L/h is very important
Temperature: 25-27°C (strain dependent)
Source:
Industrial Microbiology: an introduction (Waites et al), 2001
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Little_Ghost_again  |
In industrial reactors the pathways are non standard and the reactor is normally anaerobic, you first need to decide your scale and pathway,the above
pic is the standard pathway for say if you had a petri dish sitting on your bench top, however under anaerobic conditions the pathway would change,
you also need to consider how to eliminate the unwanted microbial growth you would get.
|
Aerobic m.o. need anaerobic conditions ? (source please)
Please, can you specify the m.o. that cause unwanted microbial growth ?
Brazialian Journal of Chemical science
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JAVA  | It's D-valine instead. 
But the parameters are now known for P. chrysogenum because I have to wait too long for get a scientific based answer in this section and
lack time: |
I'd say they're known to you because you spent time looking them up, rather than relying on one of us devoting our free labor towards the endeavor.
It's actually quite difficult to determine what specifically you are after; you only definitively and explicitly requested the biochemical pathways in
your initial post, not culture conditions.
Quote: Originally posted by JAVA  |
pH 6,5-7,7 (phosphate buffer)
oxygen supply: 25-60 mMol/L/h is very important
Temperature: 25-27°C (strain dependent)
Source:
Industrial Microbiology: an introduction (Waites et al), 2001 |
Temperature can potentially be a degree lower according to PMCID: PMC2554177, Bull World Health Organ. 1952; 6(1-2): 265–275.
Media options are available in the above as well, though it is not an industrial scaleup nor optimized for penicillin G production. Optimal
temperatures can even be a degree higher in the below source.
If you want 1) optimal production and 2) extraction methodologies, please see the temperatures, timing, culture media, pH ranges, shaker rpm (surely
correlatable to pO2 and pCO2), and solvent extractions for both, listed in
ISSN 0976-1233, Annals of Biological Research, 2012, 3 (12):5434-54
40
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by JAVA  | Quote: Originally posted by Little_Ghost_again  |
In industrial reactors the pathways are non standard and the reactor is normally anaerobic, you first need to decide your scale and pathway,the above
pic is the standard pathway for say if you had a petri dish sitting on your bench top, however under anaerobic conditions the pathway would change,
you also need to consider how to eliminate the unwanted microbial growth you would get.
|
Aerobic m.o. need anaerobic conditions ? (source please)
Please, can you specify the m.o. that cause unwanted microbial growth ?
Brazialian Journal of Chemical science |
Yes sorry I will get a source for you that relates to the two circumstances I was talking about.
Yes its aerobic m.o, but many industrial process reactors for making penicillin now use anaerobic conditions and different meadia to use other
pathways etc etc I will get you a paper for this. This is an industrial way of production.
PMCID: PMC176289
Not the best I admit but just read the papers I put in magpies thread on yeast, while they are anaerobic organisms there are reasons for culturing
them semi aerobically at times and the papers cited there explain this.
The comment on mo contamination related to my question of why he was asking the question, if he was asking so he could try and make some at home then
yes without aseptic techniques you run the risk of contamination of other microbes, I could get a citation for that.....But really? Do I honestly have
to prove that penicillin wont kill all bacteria? or that bad practice and poor aseptic technique is likely to lead to contamination??? The citation
thing is ok but thats a bit over the top. I see no reason to provide a citation or proof that aseptic precautions are needed, if there is any doubt on
that I suggest a simple google on industrial process will provide ample information.
The other point I would make is penicillin is often seen growing on old fruit at home, you also often see a white coloured fungi with it, any
conditions suitable to grow penicillin will grow other m.o.
If you read carefully what I put in my answer above I was after information. I wasnt sure if it was a research question on how industrial process was
for penicillin or if he wanted to actually make some. So I gave a mix of advice based on both circumstances, yes at home I would probably go dead
simple and aerobically, but depending on the type of penicillin industry is wanting many process are used (again google some papers for this), the
papers already posted by me in this thread give different pathways.
PMCID:
PMC2877979
Deals with improvement via different pathways amongst other things but its focus is on the technique to locate genes etc.
PMCID: PMC429153
In Vitro Activity of Penicillins Against Anaerobes
Obviously they are not going to do this aerobically as it would be pointless!
Anyway if you really do have doubts that large scale penicillin manufacture is often done anaerobically let me know and I will trawl through the mass
of papers relating to this.
Dont ask me, I only know enough to be dangerous
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I think Chemosynthesis summed the rest up pretty well.
Dont ask me, I only know enough to be dangerous
|
|
|