| Pages:
1
2
3
4
5 |
2bfrank
Harmless

Posts: 5
Registered: 11-9-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 2bfrank  | Im an IT dumbass, hence attached rather than post it directly. (been to busy to sort out how to do that, help would be good) but this mechanism was
associated with a synthetic and medicinal unit I am currently undertaking. Its predominantly using an iminium/enamine type catalyst with a Michaels
reaction and an Aldol reaction, I just thought it pretty cool. To me its poetic. Im still a little unclear, and taking my time with the stereo aspects
to it, but thought it a Pretty cool, albeit a simple mech.
Cheers |
Ive been trying to be clear how an enamine can form with just the basic catalyst. I have attached a schematic, I really would appreciate some feed
back. E1cb of an OH usually requires conjugation, If I remember correctly, but the intermediate is purely electrostatic attraction between the iminium
and the OH anion, and I do not know if its feasible. My apologize of attaching rather than somehow posting the mech.
Attachment: enamine via catalyctic base with saturated aldehyde.doc (260kB)
This file has been downloaded 1320 times
The most intelligent statements can be said so that an average person can understand, hence all the statements that require so much effort trying to
be understood, are an indicator that such a statement is not about presenting something of interest or intelligence, but rather an appearance of being
so.
|
|
|
PeetPb
Harmless

Posts: 9
Registered: 24-5-2012
Member Is Offline
Mood: No Mood
|
|
hi, I've been searching all over the internet for the mechanism of oxidation of alkanes (aryl derivatives, like toluene) to carboxylic acid by KMnO4.
I found that it should be a radical mechanism. The MnO4(-) displaces one hydrogen and makes a free radical, but beyond that I couldn't find anything
else. If you could help me I'd really appreciate it. thanx a lot
God does play dice.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PeetPb  | | hi, I've been searching all over the internet for the mechanism of oxidation of alkanes (aryl derivatives, like toluene) to carboxylic acid by KMnO4.
I found that it should be a radical mechanism. The MnO4(-) displaces one hydrogen and makes a free radical, but beyond that I couldn't find anything
else. If you could help me I'd really appreciate it. thanx a lot |
At least two mechanisms have been demonstrated that depend on the reaction media. The final product can also be determined by the reaction conditions
and media. See these studies for details:
DOI: 10.1126/science.7569922
DOI: 10.1021/ja00705a642
DOI: 10.1021/jo971168e
DOI: 10.1021/ja00904a040
For a general review of KMnO4 oxidations and their mechanisms, see: DOI: 10.1016/j.tet.2008.10.038
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
flux157
Harmless

Posts: 1
Registered: 18-2-2013
Member Is Offline
Mood: No Mood
|
|
I have stumbled on a reaction on which a 1,2-alkyl halide is converted into a ketone by the reaction with aqueous KOH. This is described in Yuki et
al, Japanese Patent 69:10,776; Chemical Abstracts 71, 30220e (1969) and also in this links: http://www.erowid.org/archive/rhodium/chemistry/tcboe/chapte... ; http://www.erowid.org/archive/rhodium/chemistry/tcboe/pictur...
| Quote: |
23.2 g (0.1 mole) of 1-(3,4-methylenedioxyphenyl)-1,2-dichloropropane is refluxed for 10hrs with 75g of 15% KOH (potassium hydroxide) solution. The
mixture is cooled extracted with benzene. The benzene is removed in vacuum and the residue distilled to yield approximately 15.2 g of piperonylacetone
(bp 149-151°C/10 mmHg). 85% yield from the dichloro compound. |
If this is true, what is the mechanism behind it? Shouldn't the reaction of a 1,2-alkyl halide with aqueous KOH give a glycol instead of a ketone? If
anyone can clear this out it would be great.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by flux157  | I have stumbled on a reaction on which a 1,2-alkyl halide is converted into a ketone by the reaction with aqueous KOH. This is described in Yuki et
al, Japanese Patent 69:10,776; Chemical Abstracts 71, 30220e (1969) and also in this links: http://www.erowid.org/archive/rhodium/chemistry/tcboe/chapte... ; http://www.erowid.org/archive/rhodium/chemistry/tcboe/pictur...
| Quote: |
23.2 g (0.1 mole) of 1-(3,4-methylenedioxyphenyl)-1,2-dichloropropane is refluxed for 10hrs with 75g of 15% KOH (potassium hydroxide) solution. The
mixture is cooled extracted with benzene. The benzene is removed in vacuum and the residue distilled to yield approximately 15.2 g of piperonylacetone
(bp 149-151°C/10 mmHg). 85% yield from the dichloro compound. |
If this is true, what is the mechanism behind it? Shouldn't the reaction of a 1,2-alkyl halide with aqueous KOH give a glycol instead of a ketone? If
anyone can clear this out it would be great. |
It would give a glycol if it were SN2, but with the big aromatic group on the 1 carbon, it will probably eliminate. Or maybe both chlorines get
substituted to form the glycol, but then elimination takes place to give 1-(3,4-methylenedioxyphenyl)-1-propen-2-ol, which would then tautomerize to
the ketone.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
cortex
Harmless

Posts: 1
Registered: 4-4-2013
Member Is Offline
Mood: No Mood
|
|
I don’t understand where the Proton in the Benkeneser Reaction(or the Birch-Reduction) with Ephedrine comes from. I found that mechanism below, is
it right? Does the proton come from the water added to stopp these reactions ?
In the normal Birch-reduction ist the alkanole acting as the donator but in the papers i found THF oder Et2O is used as a solvent and no alkanole or
anything else is used as a co-solvent.
Can somebody explain this to me ?
The mechanism i found:

best regards
[Edited on 4-4-2013 by cortex]
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by flux157  | I have stumbled on a reaction on which a 1,2-alkyl halide is converted into a ketone by the reaction with aqueous KOH. This is described in Yuki et
al, Japanese Patent 69:10,776; Chemical Abstracts 71, 30220e (1969) and also in this links: http://www.erowid.org/archive/rhodium/chemistry/tcboe/chapte... ; http://www.erowid.org/archive/rhodium/chemistry/tcboe/pictur...
| Quote: |
23.2 g (0.1 mole) of 1-(3,4-methylenedioxyphenyl)-1,2-dichloropropane is refluxed for 10hrs with 75g of 15% KOH (potassium hydroxide) solution. The
mixture is cooled extracted with benzene. The benzene is removed in vacuum and the residue distilled to yield approximately 15.2 g of piperonylacetone
(bp 149-151°C/10 mmHg). 85% yield from the dichloro compound. |
If this is true, what is the mechanism behind it? Shouldn't the reaction of a 1,2-alkyl halide with aqueous KOH give a glycol instead of a ketone? If
anyone can clear this out it would be great. |
It would give a glycol if it were SN2, but with the big aromatic group on the 1 carbon, it will probably eliminate. Or maybe both chlorines get
substituted to form the glycol, but then elimination takes place to give 1-(3,4-methylenedioxyphenyl)-1-propen-2-ol, which would then tautomerize to
the ketone. |
How is the glycol supposedly getting eliminated? That doesn't make sense to me. What I think it would happen is an elimination + substitution
reaction. The first halogen gets eliminated, and the second gets substituted, giving the hydroxyalkene which is unstable and will tautomerize to the
ketone, as you said.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Can some one help me understand or elaborate what is going on in this mechanism? What happens after the radical is formed?
Original paper
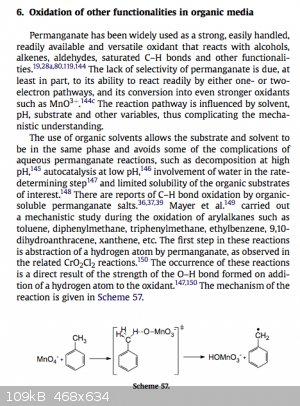
[Edited on 30-8-2014 by HeYBrO]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cheeseandbaloney  | | I recently have started teaching myself MO theory and have slowly but surely begun to understand it after drawing and building myself some molecular
orbital diagrams. Like visualizing when electron orbitals are 'out of phase' and areantibonding...etc. |
could you tell the name of the book from which you are learning.
| Quote: | but the one I have with the question was bought used online and doesn't have the solutions manual. I've looked online but the only link available to
download the solutions manual .pdf is broken.[/quote ] which book is it,i will try to find the solution manual
[Edited on 6-10-2014 by CuReUS] |
|
|
|
JAVA
Hazard to Self
 
Posts: 71
Registered: 9-1-2014
Member Is Offline
Mood: No Mood
|
|
This is the conversion of pseudoephedrine to methyl amphetamine. The intermediate that is formed is a unstable aziridine. Hence the formation of a
partial hydride that should attack on the (partial positive) benzylic carbon.
I think it's very difficult to measure this in the lab and verify the mechanism. The intermediates are so unstable that they react in nanoseconds, I
suppose.
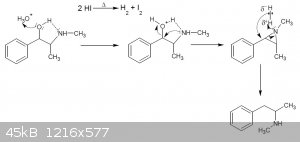
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
As far as books are concerned two were mentioned and a third would be introductory: "Pushing electrons," Daniel Weaks, sort of a first primer on the
subject. In addition I found a couple of text book chapters (attached).
Attachment: electronpushing.pdf (316kB)
This file has been downloaded 1083 times
Attachment: Pushing Electrons.pdf (9.3MB)
This file has been downloaded 1853 times
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
SecretSquirrel
Hazard to Self
 
Posts: 71
Registered: 16-4-2006
Member Is Offline
Mood: No Mood
|
|
I need help with the mechanism below. It is a Michael type addition of enamine to acetylenedicarboxylate.
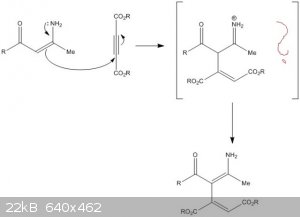
My questions are:
1.) Is the first step of the mechanism correct?
2.) If it is, what goes on electron-wise in the brackets, so that we get the end product?
Thank you very much!
[Edited on 24-9-2015 by SecretSquirrel]
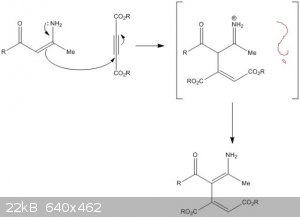
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
acetylenedicarboxylate attacks enamine (the reaction is also closely related to stork enamine alkylation), then nitrogen becomes positively charged,
while another carbon on alkyne group becomes negatively charged, thus the total charge is zero. Although I'm not sure if the last image is a correct
configuration of the molecule.
[Edited on 25-9-2015 by byko3y]
|
|
|
SecretSquirrel
Hazard to Self
 
Posts: 71
Registered: 16-4-2006
Member Is Offline
Mood: No Mood
|
|
Are you sure about that? I think it is the other was around, because acetylenedicarboxylate is electron-poor and thus cannot atack the enamine.
I think the last structure is correct... well at least acording to NMR and x-ray analyses.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SecretSquirrel  |
1.) Is the first step of the mechanism correct?
2.) If it is, what goes on electron-wise in the brackets, so that we get the end product? |
1.) It can be considered correct though it is formally wrong for two reasons. As you have it now, the equation does not hold as you made up an extra
hydrogen on the first intermediate. When the enaminone adds on the acetylenedicarboxylate triple bond, it is a nucleophilic conjugate addition, so you
need to depict the electron flow all the way to the opposite carbonyl's oxygen (you end up with a protonated amine and a deprotonated enolate, but you
can transfer the proton without actually depicting it as a separate mechanistic step - such proton transfers are considered trivial even when they are
not).
2.) The intermediate in the brackets firstly equilibrates through proton transfer (from NH2+ to O-) to give a neutral
imine form. This then equilibrates via tautomerization to give the more stable enaminone form. This can occur also by direct proton transfer from
RCOCH to O-. It is arguable what path is more likely, because this is a complex matter of proton transfer kinetics and is better
simply ignored by not depicting proton transfer steps.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
SecretSquirrel
Hazard to Self
 
Posts: 71
Registered: 16-4-2006
Member Is Offline
Mood: No Mood
|
|
Thank you!
You mean something like this? Hope I got it right this time.
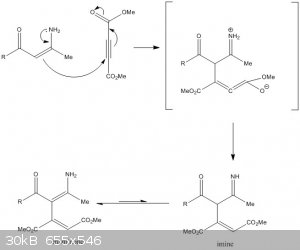
[Edited on 26-9-2015 by SecretSquirrel]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Yes, almost perfect. Just correct the geometry: the allene group should be linear.
You can further simplify by skipping the intermediate in the brackets. Like I said, proton transfers are considered trivial. Depending on the context,
they can be omitted. Sometimes even tautomerization steps can be omitted and the more stable form depicted directly, just like you did in the step
toward the imine (enol-ester tautomerization). Of course, things could become too boring as you could get away by depicting only the two reactants and
the enaminone product when omitting all the proton transfers and both tautomerizations.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Hey Guys,
So for my advanced inorganic practical course I will have to prepare Dicyclohexanephosphine. And my instructor gave me the hint to prepare for a
Grignard, the theoretical part will also be about Grignard reactions and that I will have to add the Grignard Compound to PBr3.
Makes sense. I'll probably prepare some Cyclohexanemagnesiumbromide and then make the Dicyclohexanephosphinechloride and reduce that with
LiAlH4 to the Phosphine.
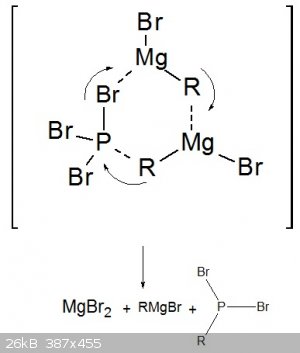
The question is how the mechanism works here. I'm pretty sure that I can remember from my lecture on Organometallics that Magnesium and Lithium
organometallic compounds tend to form sixmembered ring structures for their reactions.
So I just assumed the following mechanism. The Phosphorus has a positive partial charge due to the more electronegative Bromine atoms and the C-Mg-Br
Carbon is slightly negative so I think it could actually work like this.
I didn't care about lone pairs here so you could see everything.
Any suggestions ?
|
|
|
CaptainPike
Hazard to Self
 
Posts: 68
Registered: 21-12-2012
Member Is Offline
Mood: No Mood
|
|
I wish someone would help me to understand this often talked about, base catalyzed reaction between benzaldehyde and nitromethane. I thought I
understood that the addition of hydroxide was required to get anything started.
I wanted to perform this preparation and I used methanol as a medium (and possible phase boundary catalyst?) I made a solution of 40 mL MeOH, 20 mL
benzaldehyde, and 14 mL nitromethane, and put in the freezer. This sat, sealed in a 100 mL bottle with air in the headspace for several days in my
kitchen freezer before I was able to try the procedure.
When I added the AQUEOUS NaOH, drop wise… nothing happened. Everything was really cold so I just continued slowly until the whole, close to 9 g of
hydroxide was in. This was my idea of a 200 mmol scale reaction.
This wasn't my first time doing this and I expected to see "a bulky white precipitate", start to appear immediately. Nothing ever precipitated. I can
smell the benzaldehyde – it is clearly not gone anywhere.
The chilled down unholy trinity was pretty clear, not cloudy and definitely single phased and liquid when it went in to the reaction vessel, immersed
in a salt/ice bath to be stirred. Could the methanol have somehow decomposed the nitromethane while waiting those few days in the freezer?
I did have someone else prepare the aqueous sodium hydroxide – it acted just like I was dripping distilled water into the benzaldehyde, nitromethane
solution… Maybe there was a misunderstanding or come to think of it, discipline's GETTING PRETTY LAX AROUND HERE! I'll be seeing her later on
tonight…
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Little to nothing should have reacted until base was added. Hydroxide deprotonates the nitroalkane to the nitronate, which adds to the aldehyde and
forms a carbon-carbon bond. The negative charge is now on the oxygen.
I'm pretty sure that the nitro-alcoholate stays in solution. Slowly stir the solution into a mixture of concentrated HCl and ice. This should result
in formation of the yellow nitrostyrene, which can be filtered off.
|
|
|
CaptainPike
Hazard to Self
 
Posts: 68
Registered: 21-12-2012
Member Is Offline
Mood: No Mood
|
|
will try this, thank you
then later (the next day):
there definitely precipitated a reddish, syrupy substance after addition to the icy HCl. Decanting the supernatant, this substance was insoluble in
mildly hot pet ether. Some yellowish impurity (?) did migrate, coloring this solvent – I left the rest cool slowly… it doesn't look like anything
will be crystallizing, it still looks tarry on the bottom of the small beaker.
There is that orange peel smell again, however, which became apparent immediately on addition to the acid.
[Edited on 19-5-2016 by CaptainPike]
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
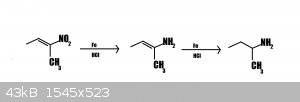
Can this work?
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
The 2nd step would tautomerize to the imine- the acidic solution would then hydrolyze it to 2-butanone.
If we don't study the mistakes of the future we're doomed to repeat them for the first time.
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
Thanks!
But is it somehow theoreticaly possible to obtain butylamine from nitrobutene by Bechamp reduction?So,after first step?Some mix of butenamine and
butylamine?
[Edited on 26-2-2017 by sulfuric acid is the king]
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
BTW how can "Lithium aluminium hydride" make third,single bond molecule,but Fe/HCl can't?
|
|
|
| Pages:
1
2
3
4
5 |