| Pages:
1
..
27
28
29
30
31
..
104 |
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I mean, I don't see any reason why it wouldn't work. Depending on the solubility of methyl ethyl ketazine in water you might lose more of your product
in the end. You could always add a base to the ammonium hydroxide solution you have and heat it, driving out the ammonia and channeling it into some
freezing cold ammonium hydroxide solution that you already have, in order to increase the concentration.
|
|
|
AlphaDecay
Hazard to Self
 
Posts: 60
Registered: 20-3-2014
Location: Uranium-238
Member Is Offline
Mood: Emitting Helium-4
|
|
I've got 2kg of aluminum sulfate laying around, but I have no idea what to do with it, considering that some reactions with it make Al(OH)3, which is
awful to deal with... http://www.sciencemadness.org/talk/viewthread.php?tid=31603#...
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by No Tears Only Dreams Now  |
I mean, I don't see any reason why it wouldn't work. Depending on the solubility of methyl ethyl ketazine in water you might lose more of your product
in the end. You could always add a base to the ammonium hydroxide solution you have and heat it, driving out the ammonia and channeling it into some
freezing cold ammonium hydroxide solution that you already have, in order to increase the concentration. |
Yeah, I was trying to avoid doing that though. I think methyl ethyl ketazine is somewhat soluble in water, but I can't find any references about
this.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi Folks,
What do you think this device did in its good days? I scavenged it from a closed analytical lab. The shiny part at the end looks like gold to me ) )
Thanks for your help!
  
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
It looks a bit like a pH probe electrode to me, though it seems a bit too complicated to be just a ph probe. Maybe someone else will have a more
convincing answer than me :p
[Edited on 3-10-2014 by alexleyenda]
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I do not think that it was used in liquid, rather measure the moisture content of some gas somehow, the small orange bulb is probably for temperature
measurement, but I can only guess. The metal on some other metallish surface reminds me of the electrical element of rectifier.
|
|
|
AlphaDecay
Hazard to Self
 
Posts: 60
Registered: 20-3-2014
Location: Uranium-238
Member Is Offline
Mood: Emitting Helium-4
|
|
Any ideas for a easy aquiring/homemade selective membrane for a potassium chloride electrolytic cell to make potassium hydroxide? I've tried gelatin
but it came off from the PVC pipe that connects the two half-cells.
[Edited on 6-10-2014 by AlphaDecay]
[Edited on 6-10-2014 by AlphaDecay]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by AlphaDecay  | Any ideas for a easy aquiring/homemade selective membrane for a potassium chloride electrolytic cell to make potassium hydroxide? I've tried gelatin
but it came off from the PVC pipe that connects the two half-cells.
|
There should be more than a few topics on this subject - some of them started by me, if I recall. Ceramic was mentioned.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Why does ATP not spontaneously react with water?
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
It does. It's why it's synthesised as needed, rather than stockpiled in the cell, because it doesn't hang around for very long.
[Edited on 9-10-2014 by Pyrovus]
Never accept that which can be changed.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Forgive me if this is a stupid question but, would an aromatic aldehyde be sufficiently acidic to react with a phenol to produce a ketone?
Specifically I'm wondering if p-dimethylaminobenzaldehyde will react with p-dimethylaminophenol to yield Michler's Ketone?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Aldehydes aren't acidic, but you don't want an acid, you want an oxidizing agent. Aldehydes aren't very good oxidizing agents, so I'd guess "no".
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Would the aldehyde be an oxidizing agent? I was hoping a reaction like the following would happen. Maybe the equilibrium could be driven by the
addition of sulfuric acid? (Sorry about the terrible picture)
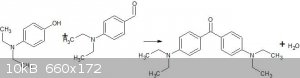
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you wanted this to happen, you'd have to use an aryl halide instead of a phenol, convert it to a Grignard, then react that with the aldehyde.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
So for amphoteric hydroxides like Cr and Cu what would happen if you attempted to react them with NaOH? Would they form Chromate/chromite or cuprate
and release ammonia or what would happen?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by bismuthate  | So for amphoteric hydroxides like Cr and Cu what would happen if you attempted to react them with NaOH? Would they form Chromate/chromite or cuprate
and release ammonia or what would happen?
|
Cr(OH)3 will react with hydroxide ion to give the soluble Cr(OH)4- ion (which can be oxidized to chromate). Zinc,
lead, and aluminum hydroxides will similarly dissolve in excess hydroxide, but copper hydroxide is barely amphoteric- you may get a bit of blue in the
solution, but you won't dissolve a significant amount of it without an outrageous concentration of base.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Yes but what would happen if I tried to dissolve the ammonia complexes of the hydroxides in base?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Tetramminecopper(II) ion is stable towards hydroxide ion (I have a book somewhere that discusses the preparation of tetramminecopper(II) hydroxide and
its use in dissolving cellulose). The chromium complex may slowly react with hydroxide to replace the ammonia ligands (Cr(III) complexes are
notoriously slow to replace their ligands, but strong base will catalyze the reaction through deprotonation of the amine ligands).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
Hello all, recently I decided to convert 10gr. of 30% palladium hydroxide on carbon to palladium II chloride dihydrate. I achieved
this by digestion of said catalyst with dilute HCl I am left with, after filtration and flash distillation, a maroon powder with a strong hydrochloric
fragrance. my question is does anyone have a suggestion of the best solvent a or duel solvent for recrystalization? Any knowledgable suggestion would
be appreciated.
|
|
|
DrMario
Hazard to Others
  
Posts: 332
Registered: 22-9-2014
Member Is Offline
Mood: Underpaid.
|
|
Sorry if I am asking a question before the one above had been answered - I waited until now, but I kind of need to know somewhat urgently: can you
suggest a method for passivating nickel? I know nickel is corrosion resistant, so "passivation" in this case is not meant as a way to protect the
nickel part, but to actually protect the chemicals in contact with nickel from its catalytic activity. These chemicals will be dissolved in a mixture
of polar solvents (including water).
One idea I had was treating nickel with oxalic acid. Nickel oxalate seems mostly insoluble in water and other polar solvents, and it is not, as far as
I know, a common catalyst, so few or no reaction would be catalyzed by it. Problem is, this layer is probably thin and not very resistant to wear.
Please suggest some better passivation materials. I'm thankful for any idea.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Is your solution acidic or basic?
Do you have to use nickel? Why not glass?
|
|
|
DrMario
Hazard to Others
  
Posts: 332
Registered: 22-9-2014
Member Is Offline
Mood: Underpaid.
|
|
Ph will be no less than 4 and no greater than 8... I think. I am not sure about the upper threshold, but the solution is likely to be mildly acidic.
Can't be glass because it has to be ferromagnetic. Nickel seemed OK, since it usually doesn't corrode easily, but, as I said, it might catalyze some
reactions.
I must note that I can, if push come to shove, deposit a thin layer of silicon oxide over the nickel by chemical vapor deposition, but I'd rather not
use that high-tech solution, if I don't have to. Besides, I am not sure how good is CVD SiO2 adhesion to nickel.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
I just made some copper "asprinate" and i can't find much info regarding its toxicity(msds)? Its not that dangerous is it? (it is a potential
medicine). Just curious.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
This page lists an LD50. I don't think it has many other hazards, I'm guessing the toxicity will be very similar to that of another insoluble
copper compound such as copper carbonate.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by gdflp  | | This page lists an LD50. I don't think it has many other hazards, I'm guessing the toxicity will be very similar to that of another insoluble
copper compound such as copper carbonate. |
Thanks! I just checked and it seems to be less toxic than aspirin itself in rats. Interesting!
|
|
|
| Pages:
1
..
27
28
29
30
31
..
104 |