| Pages:
1
..
19
20
21
22
23
..
77 |
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Great! Looks pretty  Enjoy looking at your blog! Enjoy looking at your blog!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
<a href='http://i.imgur.com/sHBDdQA' title=''><img src='http://i.imgur.com/sHBDdQA.jpg' width=800 title="I have a clearer image..."
/></a>
The pic is blurry but it's easy to see how intense the rain was: the rainbow extended below the ground, as there was enough mist on the windshield to
continue the bow.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
That just means that the pot of gold was buried as opposed to sitting on top of the ground (as it usually is).
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Doing some column chromatography with fluorescent reaction side products.




I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Thanks
Thank you volatile... I'm working on retrieving some pretty okay pictures of when i did experiments with dyes
i hope to have then on the blog soon.
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
great! I've always loved dyes work. Been reading one of the articles in sciencemadness library on them. What were you working on? I've been doing some
theorizing myself on some procedures.
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Actually I'm not working on dyes specifically it was just an interest I'd developed after I got access to some fluorescein. However, I do hope in the
future to once again dive back into dye chemistry, to be more specific though, more into fluorescent dyes and glow stick preparations. Synthesizing
organic fluorescent dyes and seeing how they behave when substituted as the main fluorescers in glow stick reactions. As well since this thread is
about pretty pictures here are the latest pictures used from my old experiment collection, just posted on www.lab-chemist.tumblr.com
Preparation of crude eosin dye by bromination of fluorescein...




Thank You......
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Sure! Those look great! Do they happen to fluoresce at all...?
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

That reddish-brown gunk is washed out with a little cold methanol and I will soon have some white crystals at the top of the funnel 
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Some Nitrogen dioxide gas and liquid at the bottom (due to pressure).

|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Yes Volatile, the crude eosin dye did fluoresce, well under the same conditions used for the fluorescein. However the colour was a deep red and I
couldn't get a good show of the colours with my camera so I don't really have a good picture to show, but this is what I did capture though...
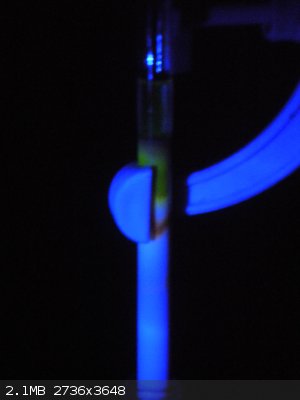
[Edited on 15-9-2014 by Ionic Chemist]
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice! Wonderful pictures! Have you considered contributing to sciencemadness wiki (the link's in my sig)? Some of your procedures would be nice to
have on there!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
PUBLIC SERVICE ANNOUNCEMENT
You can now post chemistry photos to the Rador Labs Instagram account or videos to the Rador Labs YouTube account. Links are in my signature. U2U me
for the password.
[Edited on 17.9.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Cool. What colour is the liquid? If it's blue, doesn't that mean that it's N2O3?
[Edited on 17-9-2014 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


Uranyl-nitrate hexahydrate under UV light.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Copper wire after sitting for a day in dilute sulfuric acid/peroxide solution:
 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice! (Peroxide accelerates reaction?)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Apart from nitric acid, copper metal won't react with most acids unless there is an oxidizer present(hence the H2O2). I have a single crystal of CuSO4
that I've been growing for a while; it's bigger than the end of my thumb now.
[Edited on 9-29-2014 by No Tears Only Dreams Now]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Conc. boiling H2SO4 and copper do react but in a different way. Has anybody tried this?
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
It forms sulfur dioxide and copper sulfate.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Yes but I was more trying to ask if anybody has actually tried it because it sounds like a nice experiment if anybody could tell me about procedures
and such.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was trying to figure out how this strange cloud formed with wisps going out on either side of it. There's an airplane off to the left of it for
size.

[Edited on 30-9-2014 by Morgan]
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
a moisture pocket partly rolled across the interface of two bodies of air moving in opposing directions?
|
|
|
Jylliana
Hazard to Others
  
Posts: 126
Registered: 3-10-2014
Location: The Netherlands
Member Is Offline
Mood: Bubbly ^-^
|
|

Lead(II) Iodide 
Hi, i'm new here 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Hi Jylliana!
Welcome to SM!
Hope you enjoy using the site! If you have any questions, U2U (kinda like PM-ing) someone. Nice picture, too!
I must comment, glad to see you are on the site! There are not very many females that are interested in chemistry, let alone state their gender.
Regardless of stereotypes, enjoy the site. Is there anything about chemistry you are particularly interested in? Specific branches?
-Nathan, The Volatile Chemist
|
|
|
| Pages:
1
..
19
20
21
22
23
..
77 |