| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Just a few thoughts:
1. Can you just let the calcium sulfate settle to the bottom of the vessel (it may take a few hours) then decant off a fairly clear ammonium nitrate
solution? This would save you from that messy filtration.
2. Evaporate the supernate at as high a temperature as practical until you just see signs of crystals in the body of the liquid. Then add a little
boiling water to bring back to full dissolution.
3. Then let it cool undisturbed to room temperature on its own. Don't try to rush the crystallization - that leads to fine crystals. Try
letting the supernate set at room temperature for a few hours before putting it in the refrigerator.
4. Impurities may be screwing up your crystal formation. I don't have any quick ideas for solving that problem.
I'm really not an expert at this. These are just some things I learned in organic lab. You should get some fundamental information like the
solubility curve of ammonium nitrate in water vs temperature. This may help you understand the crystallization process.
If you are saying that your cold pack is pure ammonium nitrate in water and it is behaving much differently then you may indeed be fighting
impurities. They may even be coming in through your water supply.
Try everything on a small scale until you get a good procedure.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Fatkangaroo:
50g NH4NO3 from how much Ca(NO3)2?
The difference in x-tal size would be purity, your homemade ones are not as pure as the cold pack ones... re-x from distilled water several times will
clear up some of the insoluble impurities. Then you may get some bigger x-tals.
Magpie:
Sedimentation would take a long time, speed up by cooling in the fridge but still there would be alot of CaSO4 in suspension, especially with the
large amount of precipitate filtration is a must.
The point about leaving it at room temp is also valid for large x-tal formation, the slower the solution cools the larger the x-tals will be.
-rlr
[Edited on 6-1-2005 by runlabrun]
|
|
|
Centimeter
Harmless

Posts: 28
Registered: 19-5-2004
Member Is Offline
Mood: No Mood
|
|
You're not adding nearly enough water! You want A LOT of water in order to allow for a complete reaction. If it is getting as thick as you say it
is, the chems are definately not interacting very well. You want there to be more than enough water for the calcium sulfate to settle out. The
calcium sulfate that is formed from the precipitation is most definately going to be extremely fine...meaning you would have to use real filter paper
(the slow stuff) to seperate it out. Don't try to use stoich to determine the amount of ammonium sulfate to add. Calcium nitrate is extremely
hydroscopic and you can bet that your observed masses are greatly off. Furthermore, most calcium nitrate fertilizers are a mix of ammonium nitrate and
calcium nitrate. I would recomend doing a sort of titration to determine the proper amount of ammonium sulfate. Make a solution of amonium sulfate
with known molarity and slowly add it to a solution of the calcium nitrate. Once you see that no more precipitate is forming, you have your mass
ratio! This ratio can be aplied to all future synthesis as the conditions should not change too dramatically. To purify the AN, you would have to play
around with various solvents. May I ask what you plan on using this AN for? It doesn't sound like it is for chemistry reasons or you would just
use the stuff from cold packs. If this is for explosives, I would highly recomend just using the calcium nitrate fertilizer as is. If you insist on
using ammonium nitrate, it doesn't have to be all that pure.
|
|
|
fatkangaroo
Harmless

Posts: 43
Registered: 20-12-2004
Location: australia
Member Is Offline
Mood: disturbed
|
|
runlabrun, I am using your formula every time-100 grams CN 80 gram AS and different amounts of water everytime-from this a got 50 grams AN. It is
always around the 50 gram mark although the last one from the oven was the only one I weighed. I used the other stuff for various little tests and
playing around with it trying to find out if the substance was AN.
As for information on crystal size and shape I only could find this from Urbanski.
Physical Properties.
Ammonium nitrate exists in the form of crystals, melting at 169.6'C. It occurs under five crystalline modifications distinguished by the
transition temperatures.
Tetragonal -18 - 32.1'C----Orthorhombic +32.1'C - 84.2'C---Orthorhombic 84.2'C--- Cubic 125.2'C -169.6'C---Liquid.
And on solubility from the same text.
20'C - 66.1% solubility
40'C - 73.3%
60'C - 80.2%
80'C - 85.9%
100'C - 91.0%
120'C - 94.7%
140'C - 97.4%
160'C-99.4%
Unfortunately for me 95% of that text goes straight over my head but I read it anyway just on the hope I can find the break Im after.
After reading your suggestions and hints I pretty much agree with what you have said. Probably the best thing I can to is filter off and remove as
much of the impurities as possible. This text also goes to say AN is soluble in methyl alcohol and ethyl alcohol. So tommorow I will buy a litre of
metho and try dissolving the AN into it and see if some of the junk stays back.
Thanks for you input Centimeter and runlabrun & Magpie. I will have to get some bigger jars for all that water by the sound of it. I dont know the
first thing about titration so I will have to look into it.
[Edited on 6-1-2005 by fatkangaroo]
[Edited on 6-1-2005 by fatkangaroo]
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Metho is ethyl alcohol that has been denatured with stuff so people cant (or dont want to) drink it... around here we have small amouts of methyl
alcohol and methyl iso-butyl ketone, however it is 95% ethyl alcohol....
However re-x of product in ethanol will not work.... calcium nitrate and calcium sulphate are both soluble in this solvent... you need to use water,
ice cold water to be specific... this way you wont be dissolving the contamination as well as the product, hence your re-x will work to increase
purity....
-rlr
|
|
|
fatkangaroo
Harmless

Posts: 43
Registered: 20-12-2004
Location: australia
Member Is Offline
Mood: disturbed
|
|
Taking the hint from Centimetre I added the AS to the boiling water. I scaled back the amounts to 25 grams CN and 20 grams AS. After a little stirring
the AS was fully dissolved and the water cloudy. Thinking that maybe the CN had taken in water it was put in the oven. The melting point is 45'C
so its very easy to melt which a did a couple of times.Dried it properly and added the CN to the water AS mix. As usual the mix got a milky colour but
stayed thin. For the first time I had a nice thin solution with a very fine precipitate everything was moving around and no solid gel anywhere to been
seen. Gave it a good ten minute stir and put it into the fridge. 20 minutes later the precipitate had settled still nice and thin with a inch water
layer on top.  I filtered using three slices of sheet not just one and for once
it went perfectly no blockage no need for a another filtering as it came out like water. I put the solution in the fridge and that is where it still
is.I can hardly believe the difference between doing the exact same thing but in a different order. But then again it may have been water levels all
along? So I will do this a few more times yet. I filtered using three slices of sheet not just one and for once
it went perfectly no blockage no need for a another filtering as it came out like water. I put the solution in the fridge and that is where it still
is.I can hardly believe the difference between doing the exact same thing but in a different order. But then again it may have been water levels all
along? So I will do this a few more times yet.
On a side note I bought some methylated spirits - 96% ethanol to dissolve my other molten AN in. So I put 25 grams in a jar covered in 5 times the
amount of metho gave it a good shake and it got cloudy most would not dissolve? Put 25 grams in another jar but put water in it whole lot dissolved
clear in seconds? Dont know whats happening there.
Just dried the latest batch that is the 25CN 20AS and got 22 grams of white stuff with a pink tinge back. I have since found out metho is crap
compaired to water for AN dissolving. I put the pure stuff from a cold back into metho even it would not dissove fully even after shaking it for half
an hour.
[Edited on 7-1-2005 by fatkangaroo]
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
well the reason for its insoluble nature is unknown.... NH4NO3 and CaSO4 are both alcohol soluble according to msds documents. The extent to which
they are soluble or the temperature at which the solubility occurs to a great extent may be the source of the problem, alcohols frequently have a
sharp curve on a solubility vs temperature graph whereas the same graph for water is usually more linear.
Look up msds documents to find the solubility in various organic solvents not just water.
You may need to heat the metho to overcome the solubility curve problem. However if you want to re-x to purify your ammonium nitrate ie remove the
remaining calcium sulphate i still say ice cold water as your solvent.
CaSO4 is only slightly soluble 0.21g/100g H2O at 25oC! so at 0oC where ice water is your CaSO4 will certainly precipitate, after a few runs your AN
will be pure enough to use in whatever your making it for.
If you try and use heated metho to dissolve the impure AN you will also dissolve CS and this would devoid the proceedure of any benificial outcome
other than getting the product with its impurities into solution of a common solvent.....
clear?
-rlr
|
|
|
fatkangaroo
Harmless

Posts: 43
Registered: 20-12-2004
Location: australia
Member Is Offline
Mood: disturbed
|
|
 Happy days are here! Everything is working perfectly. It all seems so simple
now. Happy days are here! Everything is working perfectly. It all seems so simple
now. 
The reason my homemade AN did not crystalize before was I had to much water in the solution. Even the cold pack AN will not crystalize out if to much
water is present. That is the longest part of this procedure - removing all that water. But thats nothing I am just happy to have this procedure down
pat. What a valuable lesson it has been. Keep in mind this was the first procedure I have ever done.
Extra thanks to runlabrun your help has been invalueble.
|
|
|
pjig
Hazard to Others
  
Posts: 179
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Ca nitrate as An repalcement for anfo?
Is this what your saying Centimeter ?
| Quote: |
If this is for explosives, I would highly recomend just using the calcium nitrate fertilizer as is. If you insist on using ammonium nitrate, it
doesn't have to be all that pure.
|
Is this even possible with the Cn 15.5-0-0 +19% calcium
only having 1% as an ammonium salt?
Im sure your referring to the CAN nitrate not the CN.... I believe CAN has around 10% or so AN in it.. Making it a weak replacement for 34% AN.
This was a study done on which nitrates had an ability to detonate with a fuel mix.
| Quote: |
At the 250 pound scale, in the standard test protocol suggested by the NRC to identify detonable materials, there is no evidence that any of the
single nitrate salts [Ca(NO3)2, KNO3, or NaNO3] were detonable with added fuel oil.3 Other fertilizers containing AN such as CAN (calcium ammonium
nitrate with 27% N) detonated with a detonation velocity ~2500 m/s
|
[Edited on 26-5-2010 by pjig]
[Edited on 26-5-2010 by pjig]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
DE178620 - http://tinyurl.com/236bu4w
Ammonium Nitrate is prepared by mixing a solution of Calcium Nitrate
with excess Ammonia and passing Carbon Dioxide into the liquid.
Ca(NO3)2 + 2NH4OH + CO2 => CaCO3 + 2 NH4NO3 + H2O
.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by runlabrun  | well the reason for its insoluble nature is unknown.... NH4NO3 and CaSO4 are both alcohol soluble according to msds documents. The extent to which
they are soluble or the temperature at which the solubility occurs to a great extent may be the source of the problem, alcohols frequently have a
sharp curve on a solubility vs temperature graph whereas the same graph for water is usually more linear.
-rlr |
When I have a question like this I take off the shelf —
Linke, William F
Suitabilities : inorganic and Metal-Organic Compounds
D Van Nostrand 1958
Me thinks your library will have a more up to date copy.
CaSO4 @ 25o C.
H2O 0.2084 gm in 100 gms solvent
Ethyl alcohol 0.314 gms 3.906% E Alcohol to 0.0029 gms
in 40.97% EtOH.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fatkangaroo  | Looking for some help to do this reaction properly as I cant seem to get it right.
As I understand it the CN is dissolved into boiling water and the calcuim drops out as it won't dissolve in water.
Well I gave it a shot and the problem is everything dissolves and there are no solids to filter off. I boiled for ten minutes or so then put it in the
frezzer. Nothing is coming out full stop.
Any help would be appreciated. |
-----------
From Google.com/patents
PROCESS OF MAKING PURE AMMONIUM NITRATE.
[From ammonium sulphate and calcium nitrate.]
US Patent 986,304. March 7, 1911
When working with dilute solutions, the gypsum formed separates
out for the greater part; the small portion remaining in solution is
precipitated by concentrating the dilute ammonium-nitrate
solution, with the exception of a very small trace, corresponding
to its solubility in solutions of ammonium-nitrate. The solution
after being separated from the gypsum and after being
sufficiently concentrated crystallizes only ammonium-nitrate upon
cooling, since the gypsum, being more soluble in the cold,
remains in solution. Starting from pure calcium-nitrate a very
pure ammonium-nitrate is therefore obtained in this way. The
commercial calcium-nitrate however always contains, besides
alumina and iron-oxid, considerable quantities of magnesia and
magnesium-nitrate as impurities. The magnesium compounds are
dissolved during the double decomposition and are not removed
by concentration. If operating with equivalent quantities of the
two salts or with an excess of sulfate of ammonia, they are upon
cooling the concentrated .solution, precipitated for the greater
part simultaneously with the ammonium-nitrate with the result
that the incombustible residue in the ammonium-nitrate is
increased to such a degree that the latter cannot be used for the
production of safety-explosives.
See complete patent for details.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fatkangaroo  | Looking for some help to do this reaction properly as I cant seem to get it right.
As I understand it the CN is dissolved into boiling water and the calcuim drops out as it won't dissolve in water.
Well I gave it a shot and the problem is everything dissolves and there are no solids to filter off. I boiled for ten minutes or so then put it in the
frezzer. Nothing is coming out full stop.
Any help would be appreciated. |
It may also be obtained, according to Ger. Pate. 166,746 and
184,144, and Fr. Pat. 465,683 of 1913, from ammonium sulphate
and sodium nitrate, either in solution or by fusion. It is best to
use excess of the nitrate: 132 kilos of ammonium sulphate and 85
kilos of sodium nitrate are dissolved in 250 litres of water and the
liquid concentrated at a temperature of 90°; after separation of
the slightly soluble sodium-ammonium salt, water is added to
form a saturated solution at 50°, the bulk of the ammonium
nitrate then separating when the liquid is cooled to 20°. It may be
obtained also from ammonium sulphate and calcium nitrate in an
autoclave at 150°.
http://tinyurl.com/3aaqhvn
------
Nydegger and Wedekind (B. P. 20907, 1909) state that
manufacture of ammonium nitrate from calcium nitrate (which by
its electrolytical production is a comparatively cheap starting-
material) has the drawback that the commercial calcium nitrate
contains various impurities, as alumina, ferric oxide, magnesia,
and magnesium nitrate. When decomposing calcium nitrate by
ammonium sulphate, the magnesium compounds enter into
solution along with ammonium nitrate, and in the evaporation
they are not removed, but remain in the crystallized ammonium
nitrate, making this useless for explosive purposes. They avoid
this by employing an excess of from i to 10 per cent, calcium
nitrate (or barium or strontium nitrate), which causes the
magnesium salts to remain in solution up to the last, after the
crystallization of the ammonium nitrate, even in cases where 10
parts MgO or upwards are present for 100 NH4NO3. From th
mother-liquor, the magnesia is recovered by an excess of
ammonium sulphate in the shape of magnesium-ammonium
sulphate. The same process is described in the Ger. P. 231394, of
Wedekind & Co.
Plantz (Ger. P. appl. P30765) gives prescriptions for obtaining pure
ammonium nitrate from crude calcium nitrate (" Norgesalpeter ").
http://tinyurl.com/2ar898o
&c., &c.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
* Ammonium Nitrate fractionally crystallizes from a solution of 8.5 parts Sodium Nitrate
to 13.2 parts Ammonium Sulfate chilled to - 15 ºC. Sodium Sulfate remains in solution.
Gas phase reaction of Ammonia with NO2 under 100 ºC
directly forms Ammonium Nitrate
2 NH3 + 2 NO2 => NH4NO3 + H2O + N2
.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I had a large amount of the fertilizer C.A.N. (15-5-0-0) product which I experimented with at length to find if it was feasible to yield quality
ammonium nitrate in bulk & with low cost or trouble. I even was going to post various yields, pics, etc, etc. However, looking back, the most
sensible thing is to find very large containers / vessels and work with several Kg units & be done with the damn thing* (after finding a method
that is utilitarian for the individual, of course).
Many years back I was really taken with hobby rocketry and thought that a pound of high quality sulfur flour and commensurate weights of KNO3 would
last as long as I would ever need it. Such was not the case. If it's really going to be used at length; it's worth making a large amount & being
done with it.
The level of hygroscopisity and deliquescense of ammonium nitrate is such that if one lives in highly humid regions or cannot use the outdoors for
other reasons, attempting large batche simply doesn't makes sense. Using an oven is so expensive that it becomes out of the question for a large scale
return. Working with single pound units, while appropriate for determining what methods may be productive; is a serious time consuming agenda for a
very common, inexpensive chemical. And while there's certainly nothing wrong with doing that once or twice for a pound or two; if one is really going
to use that chemical at length - a Kg would really only last for so long.
*Forgive me but there is a certain aspect of twisted humor in being a "Nitrate Prince"; dipping one's hands into a 5 gal bucket of crystals &
letting them run through one's fingers like a pirate with a chest of gold coins.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by franklyn  | * Ammonium Nitrate fractionally crystallizes from a solution of 8.5 parts Sodium Nitrate
to 13.2 parts Ammonium Sulfate chilled to - 15 ºC. Sodium Sulfate remains in solution. |
Any water in there,
Franklyn?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ entropy51 : The best I can do _
See bottom of pdf page 75 ( book page 49 ) here _
Manufacture of Explosives Vol I : Theoretical & Practical - 1896
http://books.google.com/books/download/The_manufacture_of_ex...
_________________
I 'm not aware that it forms a hydration adduct.
Anhydrous product always requires drying anyway , you know that.
.
|
|
|
pjig
Hazard to Others
  
Posts: 179
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Re Binders and coatings used to make AN "non-explosive"
In my latest findings I have found what seems to be even more discouraging in the coating that is used to make AN a non-explosive compound .
http://osdir.com/patents/Synthetic-resins/Anti-explosive-fer...
The coatings on the CAN are both water and hydrocarbonsoluble... Seems like they covered both basis.. My thought is create a possible
environment that can sepperate these coatings, like ph or temp.
Any Ideas? Yara really did well, in making this stuff a real pain in the assssssss!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Thinking it over there seems something '" off " about this assertion
that I quoted. Given the solubilities of Na2SO4 and NH4NO3 I would
expect the ammonium nitrate to remain in solution not the Glauber salt.
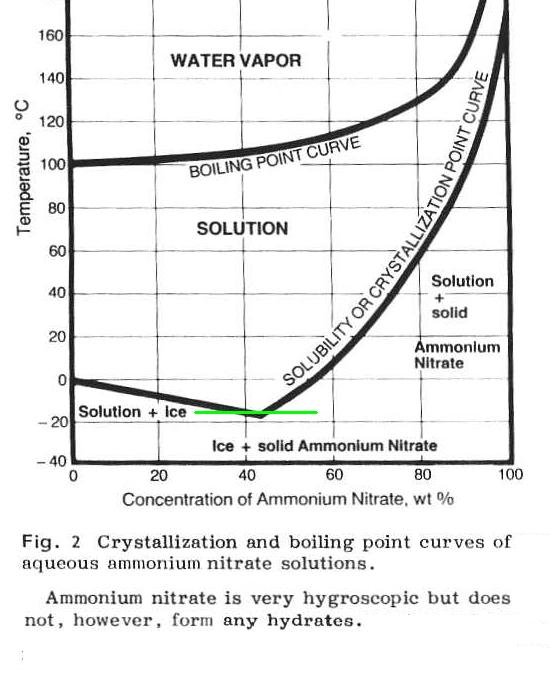 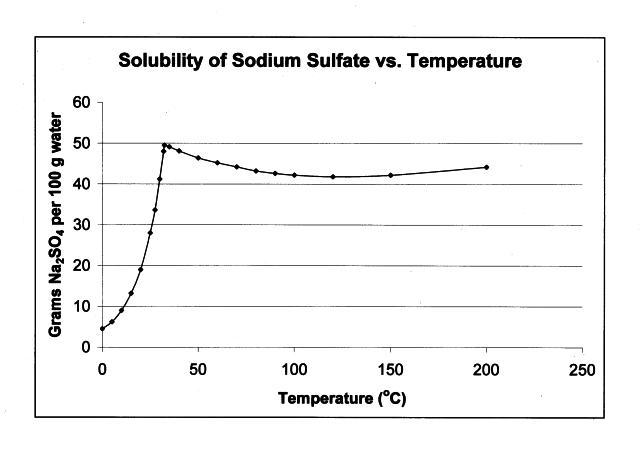 
|
|
|
midlifer
Harmless

Posts: 2
Registered: 22-12-2017
Member Is Offline
Mood: No Mood
|
|
Separating out ammonium nitrate with methanol
Just a few thoughts and opinions from experience (not deep knowledge) on this subject.
I have been experimenting with separating ammonium nitrate (AN) from both cold packs and fertilizer to use as an oxidizer in solid rocket fuel for
model rockets. (No calcium nitrate involved. That's another complication for another time. I have dealt with calcium ammonium nitrate cold packs and
have some ideas.) Both cold packs and fertilizer are coated with a substance (clay or silica) for anti-clumping purposes. No matter what you use as a
solvent, filtering with qualitative paper should leave this behind and give you a clean solution of AN. Now comes the hard part.
AN is very hygroscopic and the only way to really drive out the water of crystalization is to heat your solution for a few hours close to AN's melting
point of 169 degrees C. (How long you heat depends on how strong or weak your AN solution is. The stronger the better.) I did this using a heat plate,
a 1000 ml Pyrex beaker, and a digital thermometer. When I was satisfied it had gone far enough (bubbles actually POPPING and intuition told me it was
done), I let it cool. A few hours later, I was presented with a solid white disc of AN crystals. Hard as cement. (Dropping some into water produced
the endothermic effect.) Be careful. Do some research. Keep your eye on the temperature. Don't let it get above 169. Do this at your own risk.
However, here is a better way. Dissolve the AN in methanol directly and filter it. (Methanol is not nearly as good a solvent as water, but it does the
trick.) All the coating and most impurities will be left behind - toss them. Then put the solution in your freezer for a couple of hours after which
you will see the AN has precipitated, forming a beautiful, thick layer of crystals on the bottom of your flask. (I have never seen AN precipitate out
of water by cooling it. Perhaps I didn't have a strong enough solution. And if it does precipitate out, it still retains the water of crystallization.
I think.) Filter this methanol solution and when done lay out the filter paper to dry. In an hour or so you have dry AN with no water of
crystallization. At least that's my take on it. Correct me if I'm wrong, people.
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
You could use vacuum so that you do not need to heat the water solution to such a high temperature
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
Dissolve the AN in methanol directly and filter it. (Methanol is not nearly as good a solvent as water, but it does the trick.) All the coating and
most impurities will be left behind - toss them. Then put the solution in your freezer for a couple of hours after which you will see the AN has
precipitated, forming a beautiful, thick layer of crystals on the bottom of your flask. (I have never seen AN precipitate out of water by cooling it.
Perhaps I didn't have a strong enough solution. And if it does precipitate out, it still retains the water of crystallization. I think.) Filter this
methanol solution and when done lay out the filter paper to dry.
|
Can any point to a graph or table of solubility vs. temperature for ammonium nitrate in methanol?
Yara (and others) sell ammonium nitrate fertilizers compounded with desensitizers to prevent the use of ammonium nitrate fertilizers for ANFO, such
things as fly ash and oily stack condensate hydrocarbons from coal burning plants, along with gellants/other water activated gluey stuff to jam up
filter paper and hinder easy purification of ammonium nitrate fertilizers.
Have any tried methanol extraction on such products?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
| Pages:
1
2 |