careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Crucible Furnace Concept
I have a nifty little book "Building a Gas Fired Crucible Furnace" that gives detailed instructions for doing just that. Burning propane (and with a
suitable choice of crucible) it can melt down 20 lb of iron at a time (limited more by the practical weight you can handle than the maximum possible
crucible capacity).
This seems to be just the thing for running production loads of high temperature reactants.
I imagine adapting it to thermochemical use it would operate as a muffle furnace with a max size outer crucible (which would be essentially a
permanent fixture) and a smaller crucible inside for running the reactions, this would allow better temperature control (stick a thermocouple in the
muffle crucible) and allow atmosphere control (purge and seal the muffle crucible to keep out combustion gases), although running a reaction in just a
single crucible would also be an option.
With readily available materials temperatures of at least up to 1500C are feasible (common clay-graphite crucible rated to 1510C, silicon carbide
crucibles to 1600C, Kastalite castable refractory to 1650C). Pottery kilns seem to max out around 1300C, and many don't get that hot (1100C seems
common).
With more exotic materials and modified designs higher temperatures could be reached. High alumina castables are available up to 1850C (Refcast HA)
and pure graphite crucibles can go up to 2760C (but an oxidizing atmosphere must be avoided).
If instead of a plinth (crucible base) and outer crucible, you simply made a muffle box in one piece out of high alumina castable then you could use
pure graphite inside the controlled atmosphere and operate at 1850C. You might even be able to melt zirconium and chromium, and you could boil lead,
antimony and bismuth.
I imagine a stainless steel tube for collecting volatile product would emerge from the furnace top, and would have a heating mantle if needed to get
hot product to the condensing unit wiht clogging.
Reaction volumes on the order of a liter would be easily arranged, even a few liters, allowing processing significant amount of material.
Just an idea to kick around, and maybe pick up some refinements.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  |
With more exotic materials and modified designs higher temperatures could be reached. High alumina castables are available up to 1850C (Refcast HA)
and pure graphite crucibles can go up to 2760C (but an oxidizing atmosphere must be avoided).
|
How does thoria behave at high temperatures?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by chornedsnorkack  | Quote: Originally posted by careysub  |
With more exotic materials and modified designs higher temperatures could be reached. High alumina castables are available up to 1850C (Refcast HA)
and pure graphite crucibles can go up to 2760C (but an oxidizing atmosphere must be avoided).
|
How does thoria behave at high temperatures? |
Thoria crucibles are good to 2400C - but these days this is a non-existent product (I would be excited to find anyone anywhere selling them). Not even
Alibaba has anyone offering them.
Tungsten crucibles can do this too, and they at least exist as a product you can buy, but no one quotes prices that I can see. Like the old saw about
fancy restaurants - if you have to get a quote you can't afford it.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  |
Thoria crucibles are good to 2400C - but these days this is a non-existent product (I would be excited to find anyone anywhere selling them). Not even
Alibaba has anyone offering them.
Tungsten crucibles can do this too, and they at least exist as a product you can buy, but no one quotes prices that I can see. Like the old saw about
fancy restaurants - if you have to get a quote you can't afford it.
|
Tungsten, like graphite, vanishes in hot air. Thoria does not.
How impossible a high temperature experiment would it be to make a crucible of thoria, or some other refractory oxide like zirconia or
magnesia?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by chornedsnorkack  |
How impossible a high temperature experiment would it be to make a crucible of thoria, or some other refractory oxide like zirconia or
magnesia? |
The gas-fired furnace concept is limited by the practicality of providing a lining and muffle box/crucible wall that is affordable and can stand
direct flame exposure. You can adjust the flame to be reducing, but some tolerance to oxidation would be needed I expect.
I don't know if it is practical above the temperature of high alumina content refractories. The real value of this idea is being able to economically
process a few kilograms of material at pretty high temperatures - 1850C is quite hot. This is sufficient for lots of thermochemical processes.
If you want temperatures substantially higher then very expensive materials must be used which also cannot be cast like the pourable refractory
cements. Or else other techniques need to be considered (i.e. electric furnaces of various types), but the reaction masses will generally be much
smaller.
Zirconia would be a better bet over thoria, given that people actually sell it. Its temperature resistance seems about the same - to 2400C.
At $10/gram for thorium, a thoria liner for the furnace and muffle box is going to add up to big bucks, quite aside from issues of fabrication.
To make it out of zirconia you would need to use prefabricated zirconia pieces I think - tongue and groove zirconia bricks are available in dimensions
suitable for both the lining and the muffle box:
http://www.zircoa.com/product.coarse.grain/bricks.ring.calcu...
I have no idea how much this stuff costs.
I have had the idea for making a portable version of the crucible furnace by reducing the mass of dense refractory used - instead of the furnace body
being made of a thick refractory wall, use a ferrocement shell lined with refractory, surround that with a sheet steel cylinder creating a small air
gap for forced air circulation, then wrap that in insulating fiber matting. The strength of rebar remains tolerable up to around 1000C, so the
combination of lining and active cooling just needs to keep the shell form exceeding that temperature.
For portability it would be mounted on a metal chassis, with wheels, and detachable wheel-barrow style handles.
[Edited on 21-8-2014 by careysub]
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  |
The gas-fired furnace concept is limited by the practicality of providing a lining and muffle box/crucible wall that is affordable and can stand
direct flame exposure. You can adjust the flame to be reducing, but some tolerance to oxidation would be needed I expect.
I don't know if it is practical above the temperature of high alumina content refractories. The real value of this idea is being able to economically
process a few kilograms of material at pretty high temperatures - 1850C is quite hot. This is sufficient for lots of thermochemical processes.
If you want temperatures substantially higher then very expensive materials must be used which also cannot be cast like the pourable refractory
cements. Or else other techniques need to be considered (i.e. electric furnaces of various types), but the reaction masses will generally be much
smaller.
Zirconia would be a better bet over thoria, given that people actually sell it. Its temperature resistance seems about the same - to 2400C.
|
Melting points:
zirconia - 2715 degrees
magnesia - 2852 degrees
Certainly magnesia, even when burnt "dead" is chemically more active than zirconia. But it is not particularly expensive. How do dead burnt magnesia
refractories endure temperatures above 1800 degrees where alumina does not endure?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by chornedsnorkack  | Quote: Originally posted by careysub  |
The gas-fired furnace concept is limited by the practicality of providing a lining and muffle box/crucible wall that is affordable and can stand
direct flame exposure. You can adjust the flame to be reducing, but some tolerance to oxidation would be needed I expect.
I don't know if it is practical above the temperature of high alumina content refractories. The real value of this idea is being able to economically
process a few kilograms of material at pretty high temperatures - 1850C is quite hot. This is sufficient for lots of thermochemical processes.
If you want temperatures substantially higher then very expensive materials must be used which also cannot be cast like the pourable refractory
cements. Or else other techniques need to be considered (i.e. electric furnaces of various types), but the reaction masses will generally be much
smaller.
Zirconia would be a better bet over thoria, given that people actually sell it. Its temperature resistance seems about the same - to 2400C.
|
Melting points:
zirconia - 2715 degrees
magnesia - 2852 degrees
Certainly magnesia, even when burnt "dead" is chemically more active than zirconia. But it is not particularly expensive. How do dead burnt magnesia
refractories endure temperatures above 1800 degrees where alumina does not endure? |
How about doing some research on this, especially what is commercially available and their rated temps, and report back to us? A complete review of
high temperature ceramic option seems to be what you are after - have at it. Remember - we are talking about something someone might reasonably be
able to build with commercially available materials, at plausible cost.
[Edited on 21-8-2014 by careysub]
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Looks like the problem with magnesia is that reducing environments volatilize it above 1700 degrees. It stays involatile only if kept fully oxidized.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by chornedsnorkack  | | Looks like the problem with magnesia is that reducing environments volatilize it above 1700 degrees. It stays involatile only if kept fully oxidized.
|
I imagine that a method could be devised to ensure that your gas mixture is always oxygen-rich (for magnesia) through some sort of regulated
pre-mixing (or oxygen-poor if it is carbon).
Magnesia crucibles seem good to 2200C with proper handing. You can get one with a 2.25 liter volume for about $315:
http://ozarktech.com/otc-product/mgo-crucibles/
I particularly like the part about : "Proven performance with plutonium/uranium refining".
They also make hafnium oxide extrusions(!).
[Edited on 27-8-2014 by careysub]
|
|
|
DrAldehyde
Hazard to Self
 
Posts: 82
Registered: 12-1-2014
Member Is Offline
Mood: No Mood
|
|
I built a propane fired forge a few years ago. Kast-o-lite refractory, Reil burner, copied the basic design that can be found in many YouTube videos.
It is capable of melting iron, which I only attempted once (no pour or casting, just getting a melt). I have done quite a bit of aluminum though, at
least until the novelty wore off.The difficulties in moving from a 660c aluminum melt to a 1500c iron melt seem to increase exponentially. Unless you
have experience I would start with a simple aluminum forge, get your feet wet, then go for the high temp. Also, unless you need the volume, I would
lean towards maybe 30ml's for crucible size on the high temp unit.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by DrAldehyde  | | I built a propane fired forge a few years ago. Kast-o-lite refractory, Reil burner, copied the basic design that can be found in many YouTube videos.
It is capable of melting iron, which I only attempted once (no pour or casting, just getting a melt). I have done quite a bit of aluminum though, at
least until the novelty wore off.The difficulties in moving from a 660c aluminum melt to a 1500c iron melt seem to increase exponentially. Unless you
have experience I would start with a simple aluminum forge, get your feet wet, then go for the high temp. Also, unless you need the volume, I would
lean towards maybe 30ml's for crucible size on the high temp unit. |
You are surely correct on the best plan of how to proceed, if one wants to set up a metal melting furnace/thermochemistry furnace - start small.
The author of the crucible furnace book, Gingery, has however worked out the details of constructing one capable of 20 lb steel melts and gives a
step-by-step construction guide. If your heart is set on having such a furnace (for whatever reason), jumping right into following his guide seems
reasonable.
For me, the guide was a jumping off point to thinking about how a similar furnace could be adapted to kilogram scale high temperature production
thermochemistry (I'm never building one though).
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
| Quote: |
Tungsten, like graphite, vanishes in hot air. Thoria does not.
|
e-excuse me? dont recall that wolfram disappears when heated??
recall browsing through wolfram crucibles on alibabba some time back... i imagine there could be some interest if one person decided to buy home some
wolfram crucibles..
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  |
| Quote: |
Tungsten, like graphite, vanishes in hot air. Thoria does not.
|
e-excuse me? dont recall that wolfram disappears when heated??
recall browsing through wolfram crucibles on alibabba some time back... i imagine there could be some interest if one person decided to buy home some
wolfram crucibles.. |
Tungsten trioxide boils at about 1700 degrees, and naturally has appreciable vapour pressure below that, so tungsten heated in any oxidizing
environment is liable to volatiliza. Carbon dioxide sublimes at -78 degrees, so graphite likewise volatilizes in hot air.
Thoria is already oxidized, so it cannot react with air, and its boiling point is about 4400 Celsius, so not as volatile as tungsten trioxide. And if
it were reduced, the boiling point of metal thorium is even higher.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
hmh.. yes okay, you have a point by that
but how quickly will this substance form?
have myself considered wolfram very resistant as i once tried to destroy a small wolfram rod with electrolysis in NaCl and it didnt really seem to
care at all
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Quote: Originally posted by careysub  |
If instead of a plinth (crucible base) and outer crucible, you simply made a muffle box in one piece out of high alumina castable then you could use
pure graphite inside the controlled atmosphere and operate at 1850C. You might even be able to melt zirconium and chromium, and you could boil lead,
antimony and bismuth.
I imagine a stainless steel tube for collecting volatile product would emerge from the furnace top, and would have a heating mantle if needed to get
hot product to the condensing unit wiht clogging.
|
Well this is also my dream, building a controllable furnace for temps above 1500C. Yes, 1850C is HOT !! But melting Zr and Cr is not that simple in
open air even when you reach that temperatures. It will burn before it melts. This will only work in vacuum or inert gas furnaces, the simplest way is
flushing the vessel / crucible with Ar. N2 won't work as it will react with these metals.
And boiling Pb ? DON'T ATTEMPT or you'll intoxicate yourself and maybe others !
For high temperature hunting I tried different approach for smaller quantities: with arc melting. Temperatures of 2500C are easily reached, but only
in small quantities. MgO rods of 3mm thick melt like plastic rods of similar size despite its mp of 2800C. CaO vaporizes (and condenses in white smoke
as it cools) with a bright brick red arc color, CaC2 is easily made with CaO and charcoal, Si can be made from white sand and charcoal, I boiled
aluminum and copper (no toxic fumes). And all with a simple $100 hardware shop 150 Amps AC welder and a few carbon rods from ebay.
Disadvantage: temperature hard to control and no kilogram or liter quantities.
See my experiments at http://www.metallab.net/arcmelt.php
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by metalresearcher  |
Well this is also my dream, building a controllable furnace for temps above 1500C. Yes, 1850C is HOT !! But melting Zr and Cr is not that simple in
open air even when you reach that temperatures. It will burn before it melts. This will only work in vacuum or inert gas furnaces, the simplest way is
flushing the vessel / crucible with Ar. N2 won't work as it will react with these metals.
|
Right.
Trying to seal a crucible that is heated directly with a flame and keep its interior flushed with inert gas, while also collecting volatile product
seems a losing notion.
That as why I propose a muffle furnace (essentially a double crucible) and separate these issues. The muffle box has the inert gas flush, and
maintains the correct atmosphere for the actual reaction vessel, which has the recovery tube leading from it (through the muffle box lid) to the
collection system. It should also be much easier to control and monitor the temperature since the atmosphere inside the muffle box is more or less
static (unlike the whooshing flame) and the temperature distribution pretty even, and a thermocouple inside the muffle box tells you what heat the
reaction crucible is exposed to.
You could put a thermocouple inside the reaction crucible too, but not all reactant mixes are nice to thermocouples.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
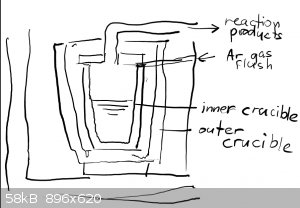
You mean two crucibles around each other like this (quick and dirty) sketch ?
Seems a nice idea, but what crucibles are you using ?
Graphite ones work fine up till 1500C and for higher you'll need MgO ones.
This is also a nice idea to get Na or K metal from their carbonates with charcoal. I tried that but the metals burnt away as I had no inert gas
flushing.
K2CO3 + 2C => 2K = 3CO
which requires only 1100-1200C but Ar flush is a strict requirement and the alkali vapors can be captured under motor oil.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
The crucible-in-a-crucible design is called a muffle or retort furnace, and they were commonly used back before industrial processes went
all-electric. Today those terms are used to simply refer to an electric furnace with atmosphere control features.
There are two ways to go about it. One is to literally get two crucibles, probably the biggest you can find/afford and then the biggest that will fit
inside of that.
The other is to build a muffle box, basically a permanent crucible that is part of the furnace, using similar methods and materials as you use to make
the furnace lining itself - probably using a castable refractory cement. Or else think of it is making your own crucible (there are many guides around
about how to do this).
The selection of materials depends on how hot you want to go. Since the muffle box/outer crucible gets the full brunt of the flame it has withstand at
least as much heat as you want for your process, and the oxidizing and/or reducing atmosphere of combustion.
Standard high alumina kiln castable refractory cements are good to 1650C:
http://www.seattlepotterysupply.com/Merchant2/merchant.mvc?S...
Higher performance alumina castables (Refcast A for example) are good to 1850C:
http://www.castablerefractories.com/high-alumina-castables.h...
Tongue and groove zirconia bricks (good to 2000C) are available to make cylindrical muffles:
http://www.zircoa.com/product.coarse.grain/bricks.ring.calcu...
Ozark Technical Ceramics has magnesia crucibles (good up to 2200C) with volumes of up to 2.25 L ($312), which can hold a smaller magnesia crucible
with a volume of 1 L ($209) :
http://www.zircoa.com/product.coarse.grain/bricks.ring.calcu...
If you are willing to keep the temperature down to a mild 1510C crucibles with volumes of 7 L or so are available for $245:
http://www.budgetcastingsupply.com/Foundry-Crucibles-s/1830....
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Just a general comment about refractory metal crucibles...(and why they are totally useless to most people)
For oxidizing atmospheres, Pt or a Pt-Rh alloy or iridium are as good as it gets. Few private individuals ever have these. Likewise for metal heating
elements, Pt is usually used, but Pt-Rh has some advantages. The price relegates these to the worthless category for most of us.
When you go to vacuum or inert gas, the metals can become star performers. Although Mo seems to be used mostly, there are W units also. But oxygen
must be rigorously excluded. It takes very good construction to meet this requirement, and realistically, most can't engineer this. This relegates
them to the worthless category for most (but not all) of us.
It isn't cheap to inert large spaces and keep them inerted. Ceramic materials will always be the materials of choice for the home scientist because of
their oxygen tolerance. It's expensive enough already to just inert the reactants.
The best commonly available high temperature ceramics (above alumina's mp) are currently Silicon Nitride, Silicon Carbide, Hafnia, Magnesia and
Zirconia. You can essentially forget about the two previous champs, thoria and beryllia. These are only available as rarities on eBay and never
cheaply.
Silicon Nitride and Hafnia are very expensive, while Silicon Carbide, Magnesia and Zirconia are less so. All can be used in oxidizing atmospheres.
I have not commented on glassy carbon as I have never looked at it in detail and I don't know enough about it to say anything.
As careysub indicated, special blends of high-performance castables are available from advanced ceramics manufactures.
[Edited on 7-10-2014 by Dan Vizine]
|
|
|
Texium
|
Thread Moved
21-11-2023 at 14:14 |