| Pages:
1
..
18
19
20
21
22
..
77 |
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mailinmypocket  | | Could it be some composition wrapped in aluminium foil and hit witnh a hammer? I've seen this done with an organic peroxide before...
|
any kind of organic peroxide,will work ?or we have to use special one?for instance will acetone peroxide wrapped in aluminium foil produce the same
effect?
|
|
|
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
HAHAHA ! homemade magnetron sputter coater !
It doesn't work well though! but looks pretty!
[Edited on 28-7-2014 by wish i had a kraken!!!]

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
NO. That would just explode.
No sparks, no visible flame. Just a Bang! Maybe some Al flakes will float to the slowly to the ground.
Yes it does.
How'd you make it?
[Edited on 28-7-2014 by Zyklon-A]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Similar but not the same actually.. Wouldn't recommend doing it though without proper safety shields as seen here
http://labphoto.tumblr.com/post/33244998226/hmtd-hexamethyle...
|
|
|
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
NO. That would just explode.
No sparks, no visible flame. Just a Bang! Maybe some Al flakes will float to the slowly to the ground.
Quote: Originally posted by wish i had a kraken!!!  | HAHAHA ! homemade magnetron sputter coater !
It doesn't work well though! but looks pretty!
[/rquote]
Yes it does.
How'd you make it?
[Edited on 28-7-2014 by Zyklon-A] |
I know it explodes wit a loud bang HAHAHAHAHAHA :-)
Dear zyklon-A I'll tell u soon about its details! for now I've got these pic for u which I designed before I make an actual one!
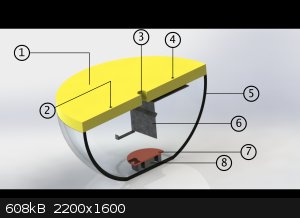
1- is plexy glass (we use this name for it here in my place)
2- is screw hole , the screw will be connected to part 7 (copper plate)
3- is hole which will be connected to vaccum pomp
4- is screw hole , the screw will be connected to part 6 (sample/substrate holder which was made whit a metal shit)
5- it's just a glass bowl but big one! HAHAHA
6- substrate holder !
7- Copper plate
8 - two ring magnets for helping the plasma to get more dens above those magnets and that area!
but it didn't coated well , I am working on it
and uh , for power supply I used an old MOT! (Microwave owen transformer) HAHAHAHAHA and a Variac
[Edited on 28-7-2014 by wish i had a kraken!!!]
|
|
|
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
Thanks really helped :-)
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Filtration doesn't get much more satisfying than this!

Filtering ferrous sulfate solution to remove insoluble impurities. I love the dramatic color change.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Hydrolysis of acetylsalicylic acid

Acetysalicylic acid, copper acetylsalicylate, and salicylic acid

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
A small thermite before mixing

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice, all of you! these pictures look great! zts, did you make it or buy it (The FeSO4)?
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I made it from
steel. In the picture, I'm filtering the carbon out of it before I recrystallized it. I then used it to make iron gall ink.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Hmmm. You could perform nucleophilic substitutions there and add different groups 
Check this: http://file.scirp.org/Html/5-1350132_33571.htm
[Edited on 12-8-2014 by Eddygp]
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
I never get tired of seeing the beauty of different things in science especially in chemistry. I recently had to sort through a few of my past
experiments and I found some old pictures from back in the day when I just started to document my experiments with fluorescein. The fluorescence still
looks amazing....
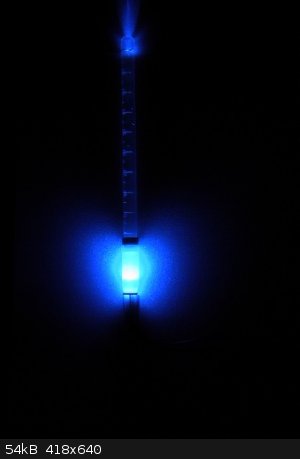 
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice pictures Ionic Chemist!
Your sig's quotes are nice, too. I always like a good chemistry quote 
Have any more pictures?!?
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Announcement
If you can, upload any photos that may be useful to the Sciencemadness Wiki - we can definitely use them!. You can hotlink your images from there
afterwards. Use the tag "img src="{insert source here}" width=800" for this.
[Edited on 25.8.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Not too many things quite like fluorescein, I always wanted to try my hand at making some for kicks. Thanks for sharing. I used to talk to a really
cool person who turned me on to chemistry and they had some really cool pictures of the stuff.
Just finished my DIY TLC lamp, so I'll have to post some pictures of some things fluorescing soon as I can find a camera to borrow.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Astro gallery NYC
I visited astro gallery in New York City with pretty cool minerals and gems exposition.. for more photos visit my blog, LINK HERE >> ASTRO GALLERY 

Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Cadmium sulfide- solid sodium sulfide was added to the right side of the dish, cadmium sulfate on the left. Such a pretty color 
 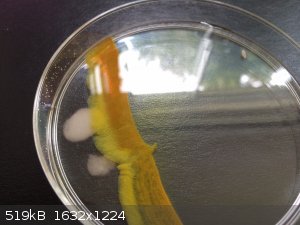
[Edited on 31-8-2014 by Mailinmypocket]
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|

Double click the image to view it full size. Took this photo of my Olympus microscope with a Nikon D3300.
[Edited on 31-8-2014 by Gooferking Science]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Here's some potassium manganate solution that I made, I also posted it here.

|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Chorination of an organic compound with elemental chlorine and UV-C light.
The glass on the left is a quartz tube with a low-pressure mercury-vapor lamp inside it what emits UV-C light with a maximum emission at 253 nm.
Normal borosilicate glass would absorb this light, but the quartz lets it through, so it could cause the chlorine molecules to break and form highly
reactive chlorine atoms.
The greenish-yellow light at the other glass joint is caused by the fluorescent reaction products.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by zts16  | | I made it from
steel. In the picture, I'm filtering the carbon out of it before I recrystallized it. I then used it to make iron gall ink. |
Ferrous sulfate is one of my favorites, along with Arkoma's copper acetylsalicylate. Do you have a write-up or recipe for making that ink? I know we
have a thread for it but I'm too lazy to go through all of that. I'm hoping to make some for a friend who enjoys calligraphy.
Oh, and Hegi, great pictures, both the landscapes and the minerals. I've really got to get around to visiting Central/Eastern Europe, for the nature
if nothing else.
[Edited on 9-1-2014 by No Tears Only Dreams Now]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Well, the ink that I made isn't the best stuff. I just kinda threw it together, loosely basing it on fiberdrunk's California live oak gall ink recipe,
which is linked in that thread. The differences with mine are that I used Texas live oak galls, because that's what I have, and more ferrous sulfate,
because for some reason it wasn't complexing properly with the amount used in the recipe. It's also still quite runny, despite stirring in several
heaping spoonfuls of acacia gum. Maybe it just needs more, I don't know. I also don't have anything to use to write with it, other than glass stirring
rods, so that probably also contributes to the messiness when writing.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by zts16  | | Well, the ink that I made isn't the best stuff. I just kinda threw it together, loosely basing it on fiberdrunk's California live oak gall ink recipe,
which is linked in that thread. The differences with mine are that I used Texas live oak galls, because that's what I have, and more ferrous sulfate,
because for some reason it wasn't complexing properly with the amount used in the recipe. It's also still quite runny, despite stirring in several
heaping spoonfuls of acacia gum. Maybe it just needs more, I don't know. I also don't have anything to use to write with it, other than glass stirring
rods, so that probably also contributes to the messiness when writing. |
Oh, all right. You said it so matter-of-factly I thought this was something you were experienced with. I'll probably have to do a fair amount of
experimentation when I finally get around to it.
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Actually I do have a few more pictures of some experiments I've conducted over the years. Some pictures are
okay and some not so much.... but I've started a blog pertaining to my overall experiments in chemistry and if anyone wants to take a look I'd be
honoured http://lab-chemist.tumblr.com/.
Apart from that here's a few of my pictures from one of my experiments. This was the chlorination of toluene to yield benzotrichloride, sadly I only
got it to produce a mixture of benzyl and benzal chloride but I still count it as a success considering the sun was my uv-source and a mirror was
improvised with some aluminum foil to help focus the light.




"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
| Pages:
1
..
18
19
20
21
22
..
77 |