| Pages:
1
..
22
23
24
25
26
..
38 |
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
got impatient with my alcohol burner so broke out the MAPP gas torch. oops. first melted a hole, then a piece popped outa this test tube after the
water ran out

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Oops indeed, poor little test tube!
I like my propane Bunsen burner for that sort of stuff. I got a pretty good deal for it online, and it just runs off of a normal barbecue propane
tank.
And I had a glassware issue two days ago When I was cleaning a bunch of glassware, I accidentally broke a 50ml erlenmeyer, getting very tiny pieces of
glass in my fingers that I didn't notice until later, one of which is still bugging me now.
[Edited on 7-20-2014 by zts16]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I broke my second to last 50mL beaker by accidentally dropping it on my hotplate....Those things don't like me!!! Luckily one was in the mail.... I
got a bunch of equipment for my b-day 
|
|
|
digitalemu
Harmless

Posts: 38
Registered: 21-10-2010
Member Is Offline
Mood: No Mood
|
|
Had brand new pair of designer jeans on that I just pulled the tags off and wearing for the first time. Picked up bottle of 4 molar H2SO4 to put it
away before I left, top disintegrated in my hand, tried to catch it with the other but failed. Smashed on the floor and splashed all up my legs. Did
not hurt me, but those jeans are done for. Fortunately nothing got above my waist and onto my good shirt or in my eyes
They tell you never to pick up a bottle by the lid, and this is exactly why. I usually wear safety goggles but was just putting something back that I
forgot to put away. Accidents happen when you are least prepared it seems.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Today I pulled a kim wipe soaked with trimethylsilylmethyl lithium out of a glove box, as soon as I opened the vacuum chamber the wipe burst into
flames.
|
|
|
mrTrifaziux
Harmless

Posts: 9
Registered: 19-8-2014
Location: Gallifrey
Member Is Offline
Mood: dehydrating
|
|
I was boiling down some weak sulfuric acid to concentrate it and accidentally spilled it all on my hotplate. Luckily there was only 20ml of liquid but
still produced room full of sulfuric acid fumes. Not fun lol
-Tr-
|
|
|
kt5000
Hazard to Others
  
Posts: 133
Registered: 27-3-2013
Location: Southwest US
Member Is Offline
Mood: Final exams
|
|
Quote: Originally posted by hissingnoise  | Adding any antibumping granules to a hot liquid is practically a recipe for bumping.
They should be in before the heat goes on. . .
|
I learned this the hard way..
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by digitalemu  | Had brand new pair of designer jeans on that I just pulled the tags off and wearing for the first time. Picked up bottle of 4 molar H2SO4 to put it
away before I left, top disintegrated in my hand, tried to catch it with the other but failed. Smashed on the floor and splashed all up my legs. Did
not hurt me, but those jeans are done for. Fortunately nothing got above my waist and onto my good shirt or in my eyes
They tell you never to pick up a bottle by the lid, and this is exactly why. I usually wear safety goggles but was just putting something back that I
forgot to put away. Accidents happen when you are least prepared it seems. |
This is why I have special clothes I wear when doing chemistry. I NEVER go into my lab unless I am wearing these clothes and this has paid off, as my
chemistry clothes now have all these holes in them.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Boron is more inflammable than I thought.
I know boron should colour flames green (Trimethyl borate certainly does) so I have had some amorphous Boron for a while and thought I would see what
colour flame it gives with an oxidant. I had some powdered potassium permanganate so I mixed a little boron powder with it using an orange stick. Bad
move. It flashed and I ended up with some nice burnt fingers. (See picture taken 2 weeks down the line). Moral of the story - Even materials not
usually thought of as very inflammable can go up when mixed with an oxidant. The flame is not noticeably green in any case.
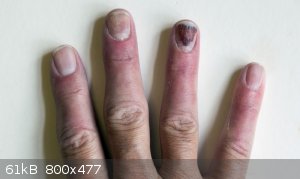
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
A picture says 1000 words.
In brief : you invented fake tan remover.
4 out of 6 fingers say so.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Oscilllator  | | This is why I have special clothes I wear when doing chemistry. I NEVER go into my lab unless I am wearing these clothes and this has paid off, as my
chemistry clothes now have all these holes in them. |
Those 'special' clothes must be quite well holed by now.
Please change your handle to:
'The Scantily Clad Chemist'
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
All summer I worked with pirhana solution and no lab coat. I'm not 100% sure how I didn't spill the stuff once but would find mysterious holes in my
shirts and pants. Clothes are impermanent and there's no reason to be dressing up for lab work.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I've been finding mysterious holes in my clothes too, despite not spilling anything on myself. I don't know for sure what causes them, but just in
case, I've started to wear only the clothes that have holes when I'm doing chemistry.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Same with me, its sulfuric acid I'm sure. I don't know how, it just happens.
[Edited on 24-8-2014 by Zyklon-A]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Zyklon-A  | Same with me, its sulfuric acid I'm sure. I don't know how, it just happens.
[Edited on 24-8-2014 by Zyklon-A] |
Actually, sulfuric doesn't fume all that much, nor does it poke holes in stuff. I did some tests with some cuts of a pair of old pants, and the
culprits turned out to be HCl and bleach.
In this case, I'd suspect nitric, as it fumes like crazy.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Interesting, I also assumed that it was sulfuric, despite it not fuming, because the holes seemed to start appearing only after I obtained sulfuric
acid. I'd used hydrochloric for months before and never noticed any holes, and I've never had any nitric acid. Your test seems pretty conclusive
though. What was the material of the pants?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I have so many holes in my clothes . . .
I wonder if I could start a new fashion trend, similar to torn jeans.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
ONce im pretty sure i nitrated a cotton shirt with N2O5.
also i get the weirdest discolorations that change color occasionally. who knows what happened there.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  |
Actually, sulfuric doesn't fume all that much, nor does it poke holes in stuff. I did some tests with some cuts of a pair of old pants, and the
culprits turned out to be HCl and bleach.
In this case, I'd suspect nitric, as it fumes like crazy. |
I have to disagree there. I once pulled apart a car battery in a stupid manner to get the sulfuric acid out, and the next day I discovered that my
trousers were COVERED in holes. There were even a couple of holes around the shoulder area of my shirt  . This was the experiment that led me to my policy regarding the clothes I wear while doing chemistry. . This was the experiment that led me to my policy regarding the clothes I wear while doing chemistry.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I also disagree, I KNOW sulfuric makes holes in things. every morning I pick up my tool belt for work, I can still see 30% through the tape-measure
pocket. and the holes in my nylon tool bag. maybe polyester? is's man-made plastic fiber for sure. they were front row for the spill. my acid fumes
just fine also. common hardware store drain cleaner.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
you should see what the battery delivery guys look like!
part of the service you get when buying new batteries is that they remove the old ones for free! they wear tyvek suits, I guess that's a bare minimum
for carrying 24 old acid filled batteries up a ladder through a small hatch and 24 new ones back down, because their clothes still have holes in them.
and we get a floor full of black spots..
all above information is intellectual property of Pyro.  |
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Hahaha, I was thinking the same thing. Not so sure how much the ladies would be in to chemical spill chic. These posts did encourage me to buy some
new pants because seriously, all of mine are holy. Not in the praise jesus way.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by smaerd  |
Hahaha, I was thinking the same thing. Not so sure how much the ladies would be in to chemical spill chic. These posts did encourage me to buy some
new pants because seriously, all of mine are holy. Not in the praise jesus way. |
Haha, when I told my parents
the other day that I needed to change out of my "holy clothes" before going to dinner, they were a bit confused at first, until I pointed out the
multiple pinholes dotting my shorts.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
The fact that so many of you seem to have sulfuric acid mist spraying all over you and burning holes in your clothes is pretty alarming. I've never
had this happen to me, ever. You guys should really review your safety procedures and how you pour and react things. Pour things slowly. Do reactions
outside. Wear a lab coat. If I ever do anything that makes a gas, I cover it with a watch glass to contain the tiny droplets that are always formed.
If something is eating through your clothes, it might be getting on your face and eyes!
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
I also only wear old clothes when in the lab not because of the chemicals themselves, but more because my lab isn't just chemistry. I find a lab coat
completly useless.
MrHomeScientist, when handling concentrated sulfuric acid there's absolutly no chance you're going to avoid holes in cotton made clothes. It just
happens. Back in the day I remember PIPPTETING a sample of Sulfuric Acid (using glass pippetes) and finding my t-shirt with those tiny holes, in the
following day. Coincidence or not, it has happened to me more than once.
|
|
|
| Pages:
1
..
22
23
24
25
26
..
38 |