| Pages:
1
2 |
Thecriscoking
Harmless

Posts: 3
Registered: 25-2-2011
Member Is Offline
Mood: No Mood
|
|
Oxidative decarboxylation with TCCA
Hello all,
I would first like to say that this is my first post to this forum, though I have been lurking for quite some time. I was not quite sure where to
post this, so the beginning section seemed proper. As I am fairly knew to this, if one finds any error in my calculations for preparing solutions,
please let me know.
So, I'll dive right in.
Oxidative decarboxylation of phenylalanine with TCCA to phenylacetonitrile
References
An Insight of the Reactions of Amines with Trichloroisocyanuric Acid
Experimental from reference
The reported procedure is representative: L -Phenylalanine (1.20 g, 7.6 mmol) was dissolved in an aq solution of 2 N NaOH (3.8 mL) and treated with
TCCA (1.17 g, 5.1 mmol) at 25 °C. After 10 min, when TLC analysis showed the complete absence of the L -phenylalanine, the reaction mixture was
treated with HCl (15 mL), followed by an aq solution of 3 N HCl (2.5 mL). After 10 min the mixture was extracted twice with Et2O (15 mL).
The organic layers were washed with H2O (10 mL), dried on Na2SO4 , filtered and concentrated in vacuo to yield
2-phenylacetonitrile (20, 0.87 g, 98%)
Actual Experiment
A 2N NaOH solution was prepared beforehand by dissolving 4 grams of NaOH into 50mL of distilled water and allowed to cool to 5°C. A 10mL solution of
3N HCl was prepared by diluting 2.9mL of conc. HCl to the specified 10mL. 1.2g of Phenylalanine was placed into a 10mL vial to which 3.8mL of the
NaOH solution was added. The solution containing Phenylalanine was allowed to sit at RT with manual stirring every few minutes until completely
dissolved. The solution was placed in the fridge until it was once again 5°C, to which it was taken out and placed in an ice bath. 1.1g of 99% TCCA
was added in portions as to prevent any runaway reactions. About half-way through adding portions of TCCA, no signs of runaway occurred, so the
remaining half was dumped into the solution. Some gas evolved, smelling of chlorine and (I believe) faintly CO2. This mixture was kept on an ice
bath for roughly one hour with manual stirring every few minutes. The mixture was removed from the ice bath and 4mL of conc. HCl was added to the
solution and the entire contents of the vial was transferred to a 125mL flask (as it was all I have to work with). The remaining 11mL of conc. HCl
(to total 15mL as suggested in the reference) was added to the 125mL flask, with some used to rinse the 10mL vial. The solution was treated with
2.5mL of 3N HCl and allowed to sit for about twenty minutes. Since no Et2O was available, toluene (2x30mL, much higher than the references
suggests) was used in place to extract from the mixture. The toluene was then dried over Na2SO4, decanted off into another
125mL flask.
This is my progress thus far, I simply need to recover the phenylacetonitrile from the toluene (granted the reaction took place).
Notes
-This was a small scale test to get the reaction down, ideally this could be scaled up with no issue.
-I have also read the WD thread pertaining to this exact experiment, but as far as I could tell, the chemist did not bother isolating the final
product.
-The phenylalanine used was both the D/L- isomers, not the L-isomer used by the reference. I felt this wouldn't be a problem, as the nitrile formed
is planar (?), void of chirality. Please let me know if this is false.
-Upon adding TCCA, I was unsure if much of it dissolved into solution. If I am not mistaken, there should be deposits of cyanuric acid when it has
reacted? Would someone please clarify this for me.
-After total addition of the acid and the evolution of gas ceased, the smell took on a very familiar aromatic smell. Hard to explain, but it reminded
me slightly of benzaldehyde.
-Upon extracting with toluene, the toluene took on a rich-piss looking color. Very yellow. When dried, it was transparent and looked like apple
juice.
-Attached are some pictures during.
After partial addition of TCCA

Complete addition of TCCA

Close-up of solution in 125mL flask after acid addition (yellow oily substance floating about)

Precipitate after addition of acid

Some questions
-Since Et2O was unavailable, would toluene be a suitable alternative? I have read that nitriles will dissolve well in nonpolars, but I was
not certain.
-I now, presumably, have a solution of BzCN in toluene. My next plan is to simply boil off the toluene, as it's B.P. is at least 100C less. Does
this seem like a liable route?
-I understand that the NaOH was used to react the TCCA with the amine, but I am still unclear of the role of HCl. Was it used to simply neutralize
the solution? If I recall correctly, no aromatic smell occurred until acid hydrolysis (terminology?).
End
----------------------
If there or any other questions I can answer or pictures of anything I may add to give some insight, please let me know. I hope I have done well in
explaining my procedure.
Attachment: An Insight of the Reactions of Amines with Trichloroisocyanuric Acid.pdf (160kB)
This file has been downloaded 969 times
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
You could try using THF as a solvent instead of Ether.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have run this reaction several times following the literature procedure. I have not achieved the yields of phenylacetonitrile claimed, in fact far
less. I do get a pleasant smelling liquid, just not much of it. Hypochlorite gives at least comparable results without the hassle of TCCA and its
by-products.
AvB
|
|
|
Thecriscoking
Harmless

Posts: 3
Registered: 25-2-2011
Member Is Offline
Mood: No Mood
|
|
I will have to purchase some of this and give it a try. Thank you for the suggestion. I will likely also purchase the ether.
Quote: Originally posted by AvBaeyer  |
I have run this reaction several times following the literature procedure. I have not achieved the yields of phenylacetonitrile claimed, in fact far
less. I do get a pleasant smelling liquid, just not much of it. Hypochlorite gives at least comparable results without the hassle of TCCA and its
by-products. |
The yield is extremely low, no? The paper states 0.87grams, at 0.786 g/mL, this should only be about 1.1 mL. Not very much, but if I obtain even a
few drops it could be chalked up as a success and maybe even scaled up. I have a good amount of Phe left, and buckets of TCCA, I may try to scale it
to maybe 50g of Phe. If literature yields are correct, that's a good 62mL of BzCN, yes?
May I ask why you suggest hypochlorite? Is it because bleach is relatively cheap? Additions of TCCA wasn't too much of a hassle, very manageable
gasses evolved.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Thecriscoking  | Experimental from reference
...
After 10 min, when TLC analysis showed the complete absence of the L -phenylalanine, the reaction mixture was treated with HCl (15 mL), followed by an
aq solution of 3 N HCl (2.5 mL). |
That is an obvious error in the article. It should state that they diluted with water (15 mL) and treated with 3M HCl (2.5 mL).
| Quote: | | The mixture was removed from the ice bath and 4mL of conc. HCl was added to the solution and the entire contents of the vial was transferred to a
125mL flask (as it was all I have to work with). The remaining 11mL of conc. HCl (to total 15mL as suggested in the reference) was added to the 125mL
flask, with some used to rinse the 10mL vial. |
Why did you add concentrated hydrochloric acid? It makes no sense and can be detrimental given that the product is hydrolysable in acidic media. There
is no mention of using conc. HCl in the article.
| Quote: | | Since no Et2O was available, toluene (2x30mL, much higher than the references suggests) was used in place to extract from the mixture. The
toluene was then dried over Na2SO4, decanted off into another 125mL flask. |
Toluene is a terrible choice. How are you going to separate it from the product?
You should use dichloromethane, MTBE, or ethyl acetate utmost. Anything higher boiling is impossible to rotavap away (unless you purge the residuals
with a lower boiling solvent - in the case of toluene methanol can be used).
| Quote: | | -Upon adding TCCA, I was unsure if much of it dissolved into solution. If I am not mistaken, there should be deposits of cyanuric acid when it has
reacted? Would someone please clarify this for me. |
The NaOH is not in excess, so there should indeed be cyanuric acid precipitating and the reaction mixture should have become slightly acidic. Carbon
dioxide should be forming. Since the media should slowly become acidic, good stirring should be used, or else you risk to have unreacted TCCA present
(especially since this is usually granulated, rather than pulverized).
| Quote: | | -After total addition of the acid and the evolution of gas ceased, the smell took on a very familiar aromatic smell. Hard to explain, but it reminded
me slightly of benzaldehyde. |
The smell of phenylacetonitrile is a bit similar to benzaldehyde, at least as far as I remember.
| Quote: | | -Since Et2O was unavailable, would toluene be a suitable alternative? I have read that nitriles will dissolve well in nonpolars, but I was
not certain. |
Phenylacetonitrile is a liquid at room temperature and is soluble in practically all organic solvents.
| Quote: | | -I now, presumably, have a solution of BzCN in toluene. My next plan is to simply boil off the toluene, as it's B.P. is at least 100C less. Does
this seem like a liable route? |
Firstly, BzCN is not phenylacetonitrile, it stands for benzoyl cyanide instead. The correct shorthand formula is BnCN or PhCH2CN.
Secondly, toluene cannot just be boiled off from a liquid. You either have to rotavap, add portions of methanol and rotavap again a few times, or
fractionally distill. There is no other simple way.
| Quote: | | -I understand that the NaOH was used to react the TCCA with the amine, but I am still unclear of the role of HCl. Was it used to simply neutralize
the solution? If I recall correctly, no aromatic smell occurred until acid hydrolysis (terminology?). |
You do not want to do any hydrolysis, if your goal is BnCN. The addition of HCl is more or less obsolete. In principle, the synthetic chemist would
want to make sure to destroy any remaining N-chloroamine, as well as assure a low pH in order to fix any basic side products in the aqueous phase
(amines such as the starting material). Dilute HCl will serve both purposes. However, the reaction mixture should already be acidic, because one
equivalent of HCl forms during the reaction.
THF is miscible with water!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
12-8-2014 at 11:05 |
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Yes. But even small amount of soluble salt (NaCl,Na2SO4,KNO3....) forces THF to salt out almost completely.
It is easy to remove toluene from benzyl cyanide by distilling it with water. Azeotrope water-toluene comes first at 85 C or so. Benzyl cyanide
distills also with water but very slowly (~1 drop per ~10 cm3 of water). So at 95-98 C distillation can be stopped because almost all toluene is then
removed.
Слава Україні !
Героям слава !
|
|
|
Thecriscoking
Harmless

Posts: 3
Registered: 25-2-2011
Member Is Offline
Mood: No Mood
|
|
Nicodem, Thank you for clearing many things up. The error in adding HCl then dilute HCl had me confused for quite some time and I did not feel
comfortable veering from the paper. The addition of the conc. HCl derived from my confusion in the paper asking for HCl then dilute HCl, where I
assumed the initial HCl (15mL) was concentrated. Toluene was the only solvent readily at hand without having to wait days for shipping and such. DCM
and Et2O will both be ordered very shortly.
A quick question: Why can toluene not be simply boiled off? I feel I am misunderstanding a key concept, as I thought due to the large difference in
b.p., BnCN would simply be left as a liquid behind (with trace amounts of toluene).
kmno4, simply adding water (assuming equimolar amounts to the toluene) will allow the water-toluene azeotrope to distill over in full (read:
relatively close to full)? I always assumed that only a small percentage formed the azeotrope. Any more information will be greatly appreciated.
I plan to scale the reaction up as soon as I get all the necessary solvents.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
In small amounts, Diethyl Ether is easily acquired. Just buy yourself a can of automotive Quick-Start fluid. I'm sure somewhere in the archives,
there will be a procedure for isolating the Ether therein.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Thecriscoking  | | A quick question: Why can toluene not be simply boiled off? I feel I am misunderstanding a key concept, as I thought due to the large difference in
b.p., BnCN would simply be left as a liquid behind (with trace amounts of toluene). |
Because as you evaporate the toluene, its molar fraction reduces, which causes its partial pressure above the liquid to drop. For this reason, it is
not possible to efficiently remove such high boiling solvents from liquid or amorphous products using a rotavapor or other evaporation devices with
limited heating and vacuum control. See Rault's law for details.
For removal of solvents like toluene from liquid products, or particularly from the annoyingly nasty resinous products, methanol can be used to purge
the residual toluene with its vapors. In practice, any lower boiling solvent can be used for purging a higher boiling solvent away, but synthetic
chemists prefer using azeotrope forming solvents expecting a better efficiency (methanol for toluene, water for pyridine, etc.).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Thecriscoking  |
kmno4, simply adding water (assuming equimolar amounts to the toluene) will allow the water-toluene azeotrope to distill over in full (read:
relatively close to full)? I always assumed that only a small percentage formed the azeotrope. Any more information will be greatly appreciated.
|
No - water should be in large (molar) excess, then all toluene is removed, leaving your nitrile (or anything high boiling) + water in flask. It is
good method for removing small amounts of solvent from heat-sensitive or/and high-boiling compounds(s), because temperatures during dist. are never
higher than 100 C.
In this way I removed traces of benzaldehyde/nitromethane from raw beta-nitrosyrene. By the way, I 'discovered' that beta-nitrostyrene also can be
distilled with water(steam), but rather in not very efficient manner: about 2-3g per 100g of distilled water.
But such product is very pure, completely stable in air, not changing its colour and getting sticky or giving off benzaldehyde.
Слава Україні !
Героям слава !
|
|
|
Reregister
Harmless

Posts: 1
Registered: 27-8-2014
Member Is Offline
Mood: No Mood
|
|
Very interesting, I've never heard about this method before.
Might it also work on Tryptophane instead or would the TCCA also oxidize the indole ring?
PS: Could be also a novel (maybe even one-pot) route to NN-Dialkyl substitutet trypamine derivates from subsituted trypamines, by simply reacting the
NN-dichloride intermediate with the corresponding alkoxide, right? (At least if the TCCA doesn't destroy the indole ring). Would be a quite funky and
easy way of NN-dialkylation.
Any thoughts about this?
[Edited on 27-8-2014 by Reregister]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
I would guess that the indole would get oxidized, probably at the 3-position. But maybe a way could be worked around it. I believe that there is a
report on Rhodium on forming the Strecker degradation product (indol-3-yl acetaldehyde) by reacting with very dilute hypochlorite. So maybe this
oxidative decarboxylation could work if similar precautions are taken. Experiment will tell.
I think alkoxide would just act as a base and form the elimination product (nitrile). I'm pretty sure that the alkylation you speak of wouldn't
(couldn't) happen. At most, the alkoxide would substitute at oxygen rather than carbon.
[Edited on 27-8-2014 by Crowfjord]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I almost have my bench built, so I figured I would have some down time.
I have Na-DCCA, and had some phenylalanine pills on hand, and wanted to attempt the oxidative decarboxylation of an alpha-amino acid. I decided to use
Na-DCCA, as opposed to TCCA, and skip using a base and see if the phenylalanine would still go into solution. It did, and did so very well! I used
about 5g of phenylalanine, and upon each addition to the Na-DCCA in water there was a lot of off-gassing and heat. The mixture started turning very
yellow, almost orangish. Even upon the first addition, an extremely nauseating smell of rose, or some other floral scent, brutally emanated from the
flask (you are seriously warned--I was also stupid for not attempting this is a closed system).
I wasn't planning on actually doing anything with the product, so I used toluene to attempt to just see if I could separate the colored oil from the
mixture, and wow, did it. The toluene took on a very deep yellow-orangish color. I wasn't in an analytical mood as it was late at night, and hadn't
weighed out anything, so the Na-DCCA could have been very much in excess. I also didn't weight the starting toluene to be able to compare it to the
final solution to determine how much had been solvated. After this, I really don't care, and don't plan on attempting it again!
The problem: As I was disposing of the experiment this morning, I became very aware of lachrymatory effects that I hadn't seen mentioned
before. Suddenly, my eyes and the skin around my nose were burning! Is it likely that an excess of Na-DCCA proceeded all the way to the chloride, or
possibly even a chloride with the same length carbon chain? Or are these effects known?
I still feel like I am covered in rose vomit! I think I might go throw up now. 
[Edited on 18-3-2015 by Loptr]
[Edited on 18-3-2015 by Loptr]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I would guess that you made some benzyl chloride as a by-product. It is a potent lachrymator, and some people can be sensitized to it and similar
compounds (Bz chloroformate is particularly bad) which make them even more potent. When you have certain benzyl groups and a chloride source, there
is a chance to make some benzyl chloride. I would guess than the cyanide can act as a good enough leaving group to be displaced by chloride, which
is also present.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I've worked with phenylacetonitrile before, and remember it has a very penetrating odour sort of in-between that of benzaldehyde and phenylacetic
acid. I tried to minimise contact with it due to the fact it is an R26 (T+) compound, and whilst I don't recall any lachrymatory effects, it was
handled appropriately in a fumehood. However, the Aldrich MSDS suggests a "burning sensation" as a symptom of exposure.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
its funny that TCCA would oxidise phenylalanine to phenacetonitrile while bleach will oxidise it to the aldehyde,I thought TCCA was much more powerful
that bleach
Quote: Originally posted by Crowfjord  | | I would guess that the indole would get oxidized, probably at the 3-position. But maybe a way could be worked around it. I believe that there is a
report on Rhodium on forming the Strecker degradation product (indol-3-yl acetaldehyde) by reacting with very dilute hypochlorite. So maybe this
oxidative decarboxylation could work if similar precautions are taken. Experiment will tell. |
here is that report
http://www.sciencemadness.org/talk/viewthread.php?tid=39224
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I thought this might be helpful if added to this content.
The Oxidation of Amino-Acids to Cyanides
H. D. Dakin. Biochem. J. 10, 319 (1916)
This paper establishes the cyanides are produced by the decomposition of previously formed dichloroamino-acids, and are as follows.
R-CH(NH2)-COOH --> R-CH(NCl2)-COOH --> R-CN + 2HCl + CO2
So it seems that an excess of hypochlorite is is required to form the dichloroamino acids, otherwise it seems aldehydes are the products.
Attachment: The Oxidation of Amino-Acids to Cyanides_Dakin.pdf (462kB)
This file has been downloaded 2492 times
[Edited on 28-4-2015 by Loptr]
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Loptr  | I thought this might be helpful if added to this content.
The Oxidation of Amino-Acids to Cyanides
H. D. Dakin. Biochem. J. 10, 319 (1916)
This paper establishes the cyanides are produced by the decomposition of previously formed dichloroamino-acids, and are as follows.
R-CH(NH2)-COOH --> R-CH(NCl2)-COOH --> R-CN + 2HCl + CO2
So it seems that an excess of hypochlorite is is required to form the dichloroamino acids, otherwise it seems aldehydes are the products.
|
Holy crap, I love that it is nearly 100 yrs old.
Good info.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by morganbw  | Quote: Originally posted by Loptr  | I thought this might be helpful if added to this content.
The Oxidation of Amino-Acids to Cyanides
H. D. Dakin. Biochem. J. 10, 319 (1916)
This paper establishes the cyanides are produced by the decomposition of previously formed dichloroamino-acids, and are as follows.
R-CH(NH2)-COOH --> R-CH(NCl2)-COOH --> R-CN + 2HCl + CO2
So it seems that an excess of hypochlorite is is required to form the dichloroamino acids, otherwise it seems aldehydes are the products.
|
Holy crap, I love that it is nearly 100 yrs old.
Good info.
|
morganbw
Yep, next year. The author refers to previous work on the subject, which would have to be even older.
Langheld [Langheld, 1909] only went as far as the monochloroamino acids, which yielded the corresponding aldehydes after hydrolysis. So if using
stoichiometric amounts of HOCL yield aldehydes by way of the monochoroamino acid, then two equivalents should result in the nitrile.
I am not sure of its reliability, but I have read in Advances in Taste-and-Odor Treatment and Control that the highest level of
phenylacetaldehyde was produced in water treatment plants when the chlorine-to-amino acid ratio was 1.5, under neutral conditions, and a reaction time
of 2 hours.
Page 85, taken from Hrudey et al. (1989)
https://books.google.com/books?id=mBnKIRVt4JIC&lpg=PA81&...
[Edited on 28-4-2015 by Loptr]
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have been reading up on this reaction for the preparation of malonic acid from aspartic acid, but I think I have found a problem with the
experimental as stated.
The procedure for phenylalanine uses two equivalents TCCA, three equivalents of NaOH, and three equivalents of amino acid. However, unless I am
mistaken, the reaction proceeds by dehydrohalogenation of a dichloroamino acid intermediate. This will end up producing six equivalents of HCl!
Wouldn't this lead to problems with the reaction going to completion, or am I missing something?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Cryolite.  | I have been reading up on this reaction for the preparation of malonic acid from aspartic acid, but I think I have found a problem with the
experimental as stated.
The procedure for phenylalanine uses two equivalents TCCA, three equivalents of NaOH, and three equivalents of amino acid. However, unless I am
mistaken, the reaction proceeds by dehydrohalogenation of a dichloroamino acid intermediate. This will end up producing six equivalents of HCl!
Wouldn't this lead to problems with the reaction going to completion, or am I missing something? |
Cyanoacetate hydrolysis to get malonic acid...nice.
Where did you get those numbers? Into the article they say into the amino acid halogenation "2/3 TCCA"...that is 3 times less than what you wrote.
The production of acid medium with TCCA is forbidden for safety reason...explosive NCl3 formation...so always with a slight exces base.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
It's two equivalents of TCCA for every three of the amino acid. That is equivalent to 2/3 mol eq of TCCA, but its easier to write it with whole
numbers.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by AvBaeyer  | I have run this reaction several times following the literature procedure. I have not achieved the yields of phenylacetonitrile claimed, in fact far
less. I do get a pleasant smelling liquid, just not much of it. Hypochlorite gives at least comparable results without the hassle of TCCA and its
by-products.
AvB |
That's interesting. I have 20 grams of phenylalanine, but I was kind of planning on using it to try to make phenylacetaldehyde by hypochlorite
oxidation and was unaware that any benzyl cyanide might be produced... do you have a literature reference for this?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Cryolite.  | | It's two equivalents of TCCA for every three of the amino acid. That is equivalent to 2/3 mol eq of TCCA, but its easier to write it with whole
numbers. |
Quoted from last page of first document:
L-Phenylalanine (1.20 g, 7.6 mmol) was dissolved in an aq solution of 2 N NaOH (3.8 mL) and treated with TCCA (1.17 g, 5.1 mmol) at 25 °C
--> amino acid 7,6 mmol
--> NaOH 7,6 mmol (2 mole/l = 2 N = 2000 mmol/l = 2 mmol/ml so 3,8 ml = 7,6 mmol)
--> TCCA 5,1 mmol (= 2/3 equivalent = 5,1 mmol /7,6 mmol)
The formed dichloride will thus be 7,6 mmol and it will set 15,2 mmol of HCl free to form the nitrile.
To neutralise this you have 7,6 mmol of NaOH what is insufficient as you stated.
Maybe that one of the product of reaction is also helping neutralization?
(-NCl-CO-)3 + H2N-CH(CO2H)-CH2-Ar --> (-NH-CO-)3 + Cl2N-CH(CO2H)-CH2-Ar
Cl2N-CH(CO2H)-CH2-Ar -2 NaOH-> 2 NaCl + N#C-CH2-Ar + CO2(g)
(-NH-CO-)3 + H2O + HCl --> NH4Cl + CO2
[Edited on 13-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by JJay  |
That's interesting. I have 20 grams of phenylalanine, but I was kind of planning on using it to try to make phenylacetaldehyde by hypochlorite
oxidation and was unaware that any benzyl cyanide might be produced... do you have a literature reference for this? |
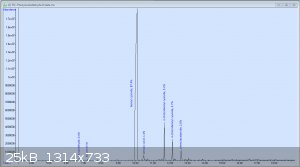
JJay, I tried the same thing hoping to get phenylacetaldehyde; I just treated a solution of phenylalanine in water with a large excess of concentrated
bleach with periodic cooling, and based on the resulting product and its odor, assumed it was the aldehyde. Until I shot in on the GC-MS at work and
discovered the following chromatogram:
|
|
|
| Pages:
1
2 |