| Pages:
1
2 |
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Titanium compounds out of TiO2
Hi everyone,
I own an amount of pure TiO2 powder, that for no surprise is very inert - it will not dissolve in boiling HCL, HNO3, etc (quite concentrated), which
is confirmed by addition of hydrogen peroxide to solution (no coloration observed). The only thing that worked a LITTLE so far was melting with
NH4HSO4 - after dilution with water the H2O2 gave intense orange-red coloration, but still only small fraction of TiO2 reacted. The goal is to prepare
some water soluble, stable to hydrolysis Ti salts, is titanium (IV) or titanyl nitrate a possibility ? If this works I plan to use it in anode
coating experiments.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
It won't be very easy, but it is possible. It does react with hot (boiling) sulfuric acid, as well as hydrofluoric acid:
TiO2+ 2 H2SO4 → Ti(SO4)2 + 2 H2O
Or:
TiO2 + H2SO4 → TiOSO4 + H2O
And:
TiO2 + 6 HF → H2TiF6 + 2H2O
http://product.lookchem.com/item/24/properties-of-titanium-d...
[Edited on 26-6-2014 by Zyklon-A]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
With the help of some of our other posters here, I dissolved TiO2 into molten NaHSO4.H2O in a test tube. The test
tube survived for at least several runs.
In short, 0.4g of TiO2 dissolved into 3.2g of molten NaHSO4.H2O to form a transparent yellow melt. Upon dissolution
into cold water, no residue was noted.
http://www.sciencemadness.org/talk/viewthread.php?tid=27624&...
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
What soluble product exactly did it form, WGTR? Can I really use NH4HSO4 instead? Also - if I manage to dissolve it, is it possible to get it back as
a hydrated oxide or even carbonate with NaHCO3 solution, since I want to prepare nitrate, will it be gel like or powder that can be filtered?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
In the presence of water, it's more like TiOSO4. If a weak solution is refluxed for a hour or two, the salt hydrolyzes, precipitating out
some amorphous hydrated form of titania. This wet precipitate will still have some sulfate in it, but will generally be easier to react with other
concentrated acids than your (likely) dead-burned powder. Good luck trying to filter it. It needs to be precipitated out, either with a
centrifuge, or by letting it sit for a day or so. If it doesn't settle out, then perhaps dialyzing the solution and/or heating it gently will help:
http://www.sciencemadness.org/talk/viewthread.php?tid=27624&...
I have no idea whether ammonium bisulfate will work or not. I got the sodium bisulfate at a pool supply store. I'm really a one-trick pony when it
comes to titanium chemistry. I haven't done much with it.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@papaya:
Dissolving TiO2 is a hard nut to crack, although it depends a lot on what grade it is.
My latest batch of food grade Anatase didn't dissolve even the slightest little bit on 3 hours of boiling with 98 % H2SO4!
Other grades will be slightly more amenable to dissolution with H2SO4. HF is of course out of the question for most amateurs.
Fusion with NaHSO4 will work with some grades. It will work better than NH4HSO4.
With my stubborn Anatase I resorted to fusing with twice the weight in KOH. This forms potassium titanate, which after cooling and on treatment with
plenty of water then hydrolyses to TiO2:
K2TiO3(s) + H2O(l) === > 2 KOH(aq) + TiO2(s)
But this freshly prepared TiO2 (Ti(OH)4?) is much, much more soluble in H2SO4.
The salts of Ti(IV) are all titanyl salts, as far as I now: titanyl sulphate for instance is TiOSO4 = TiO<sup>2+</sup> +
SO<sub>4</sub><sup>2-</sup>. These salts are very prone to hydrolysis and I'm not sure they can even be isolated from their
solutions by straightforward methods (like evaporation).
[Edited on 26-6-2014 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | These salts are very prone to hydrolysis and I'm not sure they can even be isolated from their solutions by straightforward methods (like evaporation)
|
CHRIS25 can do this. Oh yes. I'm a Believer.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And I need proof.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Can't prove or disprove a Belief, which is VERY convenient (for me) !
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Damn aga you sure know how to go offtopic sometimes. And yes I know my post doesn't help that at all.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Papaya, this should answer your questions:
Analytical chemistry, Volume 1, Frederick Pearson Treadwell, William Thomas Hall - J. Wiley & sons, inc., 1921
 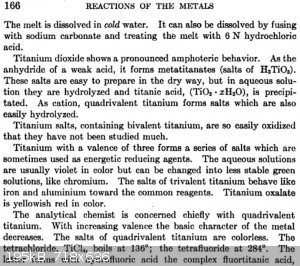 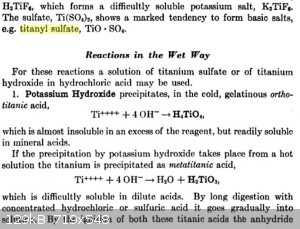   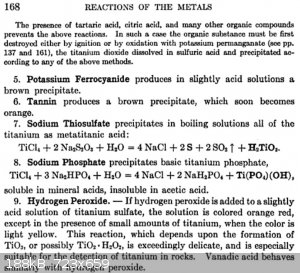 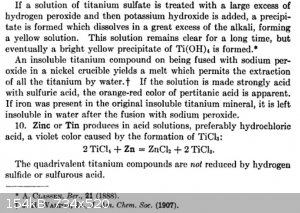  
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Thanks WGTR, quite informative. So it appears that after fusion one gets titanium sulfate, but then
https://www.sciencemadness.org/whisper/files.php?pid=335947&...
it's transformed inti titanyl in solution?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It seems the Ti(IV) salts can only exist in solution as titanyl salts (solutions of). Pure TiCl<sub>4</sub> is a covalent, liquid (STP)
compound, not an ionic solid.
Ti(III) salts like TiCl<sub>3</sub> and Ti<sub>2</sub>(SO<sub>4</sub> <sub>3</sub> do appear to exist as salts of
[Ti(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>. Some can be isolated, or so I've read. The (III) sulphate forms
alums with Cs and Rb, possibly with NH4. <sub>3</sub> do appear to exist as salts of
[Ti(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>. Some can be isolated, or so I've read. The (III) sulphate forms
alums with Cs and Rb, possibly with NH4.
[Edited on 27-6-2014 by blogfast25]
[Edited on 27-6-2014 by blogfast25]
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Maybe it can be useful:http://titanium.atomistry.com/titanium_dioxide.html
Its about titanium dioxide and other titanium compounds that you can't find anywhere else.
[Edited on 28-6-2014 by sasan]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Would Ammonium Bi-Fluoride be strong enough for a route to the fluoride salt and then be further displaced to whatever salt you're trying to form?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hyfalcon  | | Would Ammonium Bi-Fluoride be strong enough for a route to the fluoride salt and then be further displaced to whatever salt you're trying to form?
|
That's a good question. NH4HF2 isn't very suitable for fusions because of it low MP and tendency to decompose (into NH4F + HF!) when heated. It's
certainly something that should NEVER be attempted outside of a good fume hood.
So that leaves using it in concentrated solution. I've used 50 w% NH4HF2 solutions to dissolve freshly prepared Zr(OH)4 and that's not easy
either despite the fact that zirconium (IV) forms a strong complex with fluoride ions (hexafluoro zirconate anions). That dissolution requires some
excess of NH4HF2 and modest heating.
For that reason I think attacking hard calcined TiO2 with NH4HF2 will probably not work so well. I could be wrong on that.
Freshly prepared hydrated titania ("Ti(OH)4") however would be quite responsive to NH4HF2, yielding (NH4)2TiF6 (or variants thereof). The problem is
then to displace the fluoride ions with whatever you fancy: as the hexafluoro titanate complex is a strong one, that will not be easy either.
[Edited on 28-6-2014 by blogfast25]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
HF and similar things, though available to me, aside from being toxic are inconvenient since you must forget about GLASS vessels when working with
them (or not? )
I retried fusion with NH4HSO4, this time I held it longer at high T - it's shitty, the melt bubbles and decomposes after(!) addition of TiO2 with
white fumes and strong AMMONIA(!) odor (at lower temp it turns to solid). At the end I obtained yellow colored hard porous chunk, that partially
dissolved in cold water and most if not all TiO2 precipitated at once. H2O2 test gives yellow color this time (not red :O ), but I think 99% of TiO2
is not dissolved. I don't have any NaHSO4 to do it right way for now...
Btw. red peroxide compound formed with Ti4+ solution seems very stable - it's still the same color after few days - any information about known Ti
peroxo complexes ? is it similar to CrO8 (3-) anion? Would be a nice goal to prepare and isolate such a compound in free state.
[Edited on 28-6-2014 by papaya]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  | | At the end I obtained yellow colored hard porous chunk, that partially dissolved in cold water and most if not all TiO2 precipitated at once. H2O2
test gives yellow color this time (not red :O ), but I think 99% of TiO2 is not dissolved. I don't have any NaHSO4 to do it right way for now...
|
I've no idea whether any titanium/titanyl sulphate formed in your experiment but if it did then dissolving the frit in water is not a good idea: it
causes any titanium (IV) to promptly hydrolyse (to hydrated titania). Ti (IV) compounds need a very low pH to stay in solution (except for hexafluoro
titanates).
Try once more but this time try and dissolve the frit in 30 % H2SO4.
You could also try and treat that first precipitate with conc. H2SO4 to see if you get any dissolution.
[Edited on 28-6-2014 by blogfast25]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by papaya  |
I retried fusion with NH4HSO4, this time I held it longer at high T - it's shitty, the melt bubbles and decomposes after(!) addition of TiO2 with
white fumes and strong AMMONIA(!) odor (at lower temp it turns to solid). At the end I obtained yellow colored hard porous chunk, that partially
dissolved in cold water and most if not all TiO2 precipitated at once. H2O2 test gives yellow color this time (not red :O ), but I think 99% of TiO2
is not dissolved. I don't have any NaHSO4 to do it right way for now...
[Edited on 28-6-2014 by papaya] |
I couldn't find the same sort of information on titania, but here is what happens when magnesia is reacted with molten
ammonium pyrosulfate (which is what you have when you heat up your ammonium bisulfate ). Note that ammonia is given off:
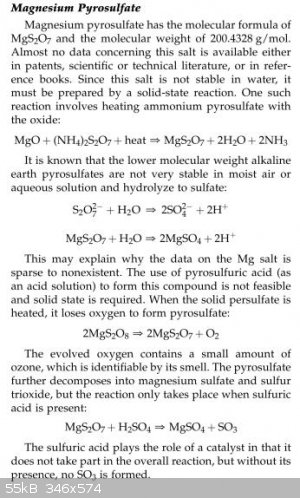
Encyclopedia of the Alkaline Earth Compounds,
Richard C. Ropp
Newnes, Dec 31, 2012
Potassium pyrosulfate (or bisulfate) is recommended over sodium pyrosulfate, as it appears that the sodium salt gives off
SO3 more readily on overheating. However, either one can be used it care is taken when heating. I used the
sodium salt when fusing TiO2 in a test tube, and it worked fine. Sodium bisulfate is used to lower pH in pools, and
can be found in practically every hardware or pool supply store. It dehydrates to sodium pyrosulfate after fusion.
Attachment: Vries69thermal.pdf (259kB)
This file has been downloaded 499 times
Whatever salt you use, when dissolving the TiO2 the melt should be gently heated until all the titania dissolves to a
clear, transparent melt. If this doesn't happen, there is probably not enough extra pyrosulfate salt in the melt. Titanium
sulfate melts at a very high temperature (not sure of the exact number), and extra pyrosulfate is needed to ensure that
everything stays molten at a sufficiently low temperature.
Also, titanyl sulfate does tend to hydrolyze in water, but I didn't notice it happening too quickly at room temperature. This may
have been the result of the excess bisulfate left over from the melt. I stored 10% stock solutions of the previously fused mass
into water. Over a period of weeks only mild decomposition was noticed.
Edit:
On the other hand, if you can dissolve TiO2 into a sufficient excess of molten ammonium pyrosulfate, perhaps the excess
ammonia could be removed more easily than the extraneous metal salts could, perhaps by extended heating above 400-500C
(Attachment: t091a25-1.pdf (258kB)
This file has been downloaded 491 times).
[Edited on 6-29-2014 by WGTR]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  | [
Also, titanyl sulfate does tend to hydrolyze in water, but I didn't notice it happening too quickly at room temperature. This may
have been the result of the excess bisulfate left over from the melt. I stored 10% stock solutions of the previously fused mass
into water. Over a period of weeks only mild decomposition was noticed.
|
Yes, with excess bisulphate left the pH will have been kept low. Also, 10 % TiOSO4 is fairly low. In the older sulphate process, titania was
precipitated from titanyl sulphate/ferrous suphate solutions essentially by strongly diluting with water: dilute the acid reserve and the hydrated
titania drops out.
Your remarks on potassium pyrosulphate remind me of another reaction reported here by 'plante' and 'garage chemist': heating a mixture of TiO2, NaCl
and potassium pyrosulphate yields vapours of TiCl4!
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
And if you were to produce TiCl4, you could reduce that with aluminum or preferably hydrogen to make TiCl3, which would be an
easy feedstock for other titanium compounds.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | | And if you were to produce TiCl4, you could reduce that with aluminum or preferably hydrogen to make TiCl3, which would be an
easy feedstock for other titanium compounds. |
TiCl4 is very hard to handle though: all moisture has to be avoided or you've got a smoke bomb on your hands.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Yeah, I guess you'd have to dry your reaction vessel extremely well and then channel the TiCl4 gas into another vessel where it reacts with
hydrogen, and then TiCl3 would crystalize in there. At least I think that would be a possible way to go about it. It's all purely
speculative on my part. It would be VERY cool to see two colorless gases combine and form a purple solid.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Actually, in spite of claims on how hard it is to attack TiO2, the protective layer on Titanium metal, some exciting research that suggests otherwise!
Per this source, a noted manufacturer of resistance Titanium alloys (see this source https://www.google.com/url?sa=t&source=web&rct=j&... ), there are a couple of paths that even an a home chemist may wish to explore.
First, and most exciting, if one were to scrap (that is, mechanically damage) some mildly heated Ti metal in an atomsphere of very dry (and mostly
oxygen free) chlorine gas, the metal may even start to burn! To quote from material prepared by Titanium Metals Corporation:
"Dry chlorine can cause rapid attack on titanium and may even cause ignition if moisture content is sufficiently low (Table 2).(3)However, one percent
of water is generally sufficient for passivation or repassivation.."
A cooled area of a vessel in which this ignition occurs could collect, most likely, anhydrous Titanium chloride, but I am not sure how practical this
is really for the home chemist or for any large scale work.
Based on essentially the same chemical reaction and electrochemistry (my guess, some galvanic corrosion also) concentrated aerated solutions of AlCl3
and ZnCl2 appear to corrode the metal as well.
I must explore some of these paths when I acquire the metal.
[Edited on 30-6-2014 by AJKOER]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
AJ:
The metal dissolves in hot 37 % HCl and similar concentration hot H2SO4 quite easily, as I've demonstrated on this forum a few times. For the Ti(III)
sulphate concentrations of 30 w% and higher can be achieved (personal experience) in a matter of 30 mins to 1 hour. But that says NOTHING about highly
calcined commercial TiO2 and how it responds to acids.
Anhydrous TiCl4 is hard to handle, no matter how you prepare it.
Titanium powder ('sponge' as it's called) is readily available from eBay sellers, not expensive either. Pyros use it to produce white sparks of
burning Ti particles. Direct union with oxygen or chlorine is probably possible at higher than RT temperatures.
|
|
|
| Pages:
1
2 |