Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Synthesis of Arecoline - 3 Steps from Nicotinic Acid
<div style="text-align: center;"><span style="color: Red;">
**Caution! Iodomethane is a probable carcinogen and is highly volatile!**
**Caution! Arecoline is a possible carcinogen and is biologically active!**</span></div></strong><br />
Arecoline is an alkaloid natural product found in the fruit of the Areca palm, Areca catechu. The fruit, known colloquially as betel nuts, is used recreationally in various Pacific and Asian cultures for its mild stimulant properties, and is also used in Ayurvedic medicine
and traditional Chinese medicine. Arecoline is a muscarinic acetylcholine M1 and M2 receptor agonist, and has generated moderate interest in academic
research for use in Alzheimer's and other neurodegenerative conditions, though its application is hindered by the compound's reported carcinogenicity
(see Wikipedia). Additionally, the strucuture-activity relationship of arecoline analogues is of continued interest in medicinal
chemistry.[1, 2, 3] While arecoline itself is commercially available and relatively inexpensive, its synthesis is presented here as
an 'intermediate' level synthesis, and an extremely brief 'total synthesis'. This route utilizes a Fischer esterification,[4] N-methylation and semi-reduction using in situ generated sodium
triacetoxyborohydride.[5] Arecoline hydrobromide was obtained 11% overall yield starting from nicotinic acid - no attempt was made to optimize yield.
Discussion
The Fischer esterification of nicotinic acid proceeds in good yield provided sufficient sulfuric acid is present, and provided the reaction is allowed
to run long enough. N-methylation proceeded uneventfully in excellent yield. Though literature preparation of N-methyl pyridinium salts
generally call for toluene and/or heating, this reaction was found to proceed in acetone at room temperature overnight. The subsequent semi-reduction
of this methylpyridinium salt to the tetrahydropyrdine has also been reported using sodium borohydride in biphasic benzene/water
[6](lit. 42%), though the purported yield with sodium triacetoxyborohydride is higher (lit. 67%). Sodium triacetoxyborohydride is a
mild reducing agent, and will selectively reduce iminium species and aldehydes. Note that the pyridinium species produced here could be considered an
"ene-iminium" ion - upon reduction of the first iminium, the enamine can tautomerize to the iminium and undergo reduction. Sodium borohydride is not
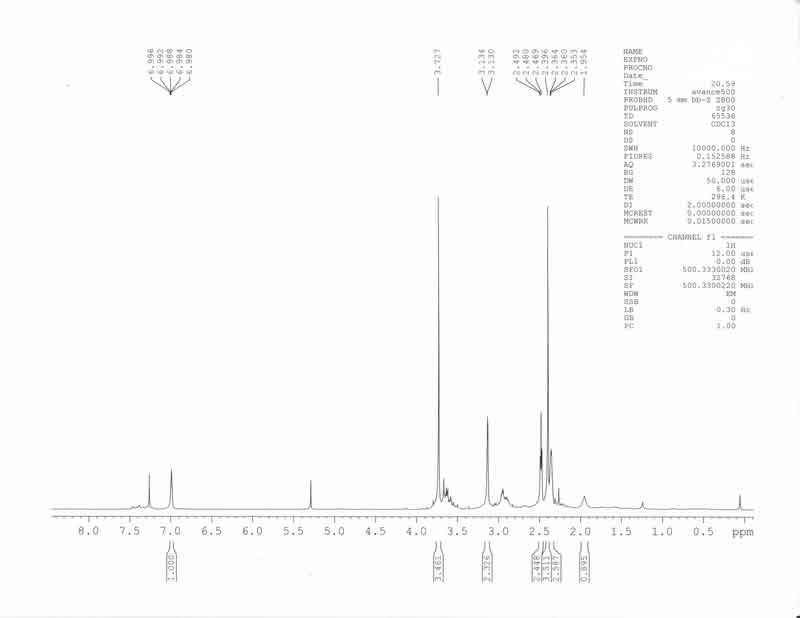
expected to provide this selectivity, and is more likely to give methyl 1-methylpiperidine-3-carboxylate as a by-product. The crude 1H-NMR of the
triacetoxyborohydride reduction indicated good conversion to product, but fractional crystallization of the product as the hydrobromide salt proved
difficult. Modification of this purification step would likely improve yield dramatically. It is uncertain whether the hydrochloride salt is
comparable to the hydrobromide produced here. On a small scale (<1g), this reduction proceeded in >50% isolated yield.
Experimental
Methyl Nicotinate
Nicotinic acid (25g, 203mmol) was suspended in methanol (75ml) and sulfuric acid (30ml) was added cautiously over ~1 hr. The now homogeneous mixture
was refluxed for 2 hrs, cooled to room temperature, poured into chipped ice and basified to pH >10 with solid potassium carbonate <span
style="color: Red;">(**Caution! A large amount of CO2 is evolved**)</span>. The basic solution was extracted with ethyl acetate (3x 75ml),
and the combined organic phase was dried over magnesium sulfate, filtered and concentrated to give methyl nicotinate as a lightly colored oil which
solidified upon standing (20.40g, 73%).
3-(methoxycarbonyl)-1-methylpyridinium iodide
Methyl nicotinate (20.40g, 149mmol) was dissolved in acetone (200ml), the solution was cooled to 0ºC and iodomethane (28ml, 447mmol) was added with stirring (no exotherm observed). The reaction vessel was wrapped in aluminum foil, andthe reaction was
allowed to warm to room temperature and stand overnight. The thick suspension was filtered, rinsed with acetone, and dried in vacuo to give
the title compound as a light yellow, hygroscopic solid (37.57g, 91%). This material was used without purification, but may be recrystallized from
ethanol.
Methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate hydrobromide
3-(Methoxycarbonyl)-1-methylpyridinium iodide (37.57g, 135mmol) was dissolved in a mixture of methanol (150ml) and glacial acetic acid (80ml) and
cooled to 0ºC. Sodium borohydride (10.21g, 270mmol) was added in portions over ~1 hr <span style="color: Red;">(**Caution! A large amount of
H2 is evolved! Hydrogen is extremely flammable!**)</span> The reaction mixture was an intense orange color (see below). The mixture
was stirred for 3hrs, then quenched with water (50ml), washed with diethyl ether (2x 75ml), basified to pH >10 with solid NaOH while cooling in an
ice bath, and extracted with DCM (3x 100ml). The combined DCM extract was dried over MgSO4, filtered and concetrated to give 17.65g of a red oil (84%
crude, see NMR below). This oil was dissolved in water (ca. 100ml) and acidified to pH ~4 with hydrobromic acid. The water was evaporated in
vacuo to give a red-brown foam. The residue was dissolved in hot ethanol, and diethyl ether added to induce crystallization. Only one crop of
crystals could be obtained, which was recrystallized from ethanol/ether to give the title compound as a light yellow crystalline solid. Structure was
confirmed by NMR and melting point (4.97g, 16%. MP 167-172ºC).

Sodium triacetoxyborohydride reduction

Purified product
Spectra
1H-NMR crude free base (CDCl3)
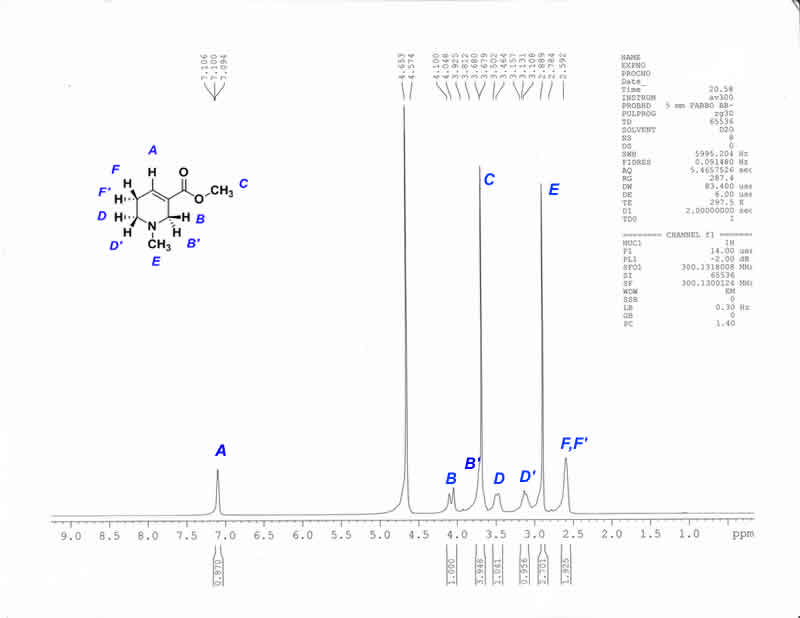
1H-NMR Purified Hydrobromide (D2O)
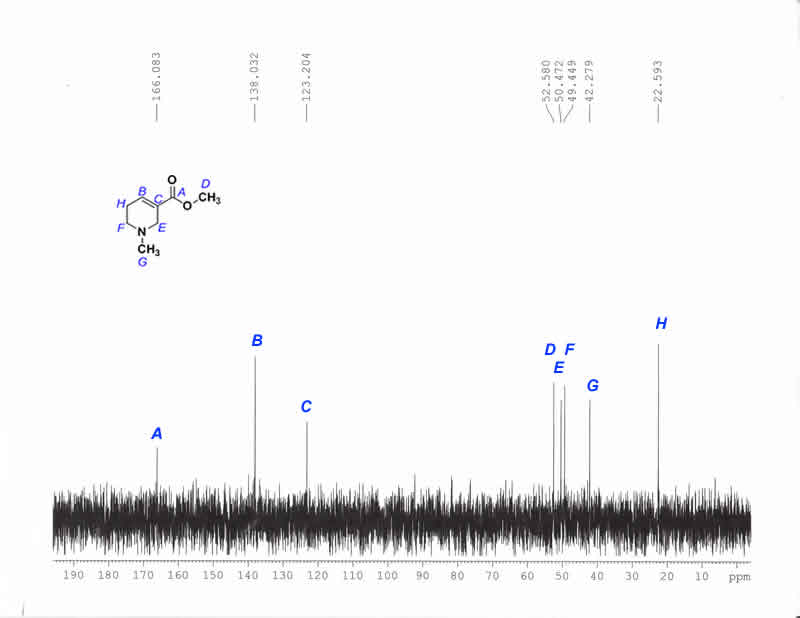
13C-NMR Purified Hydrobromide (D2O)
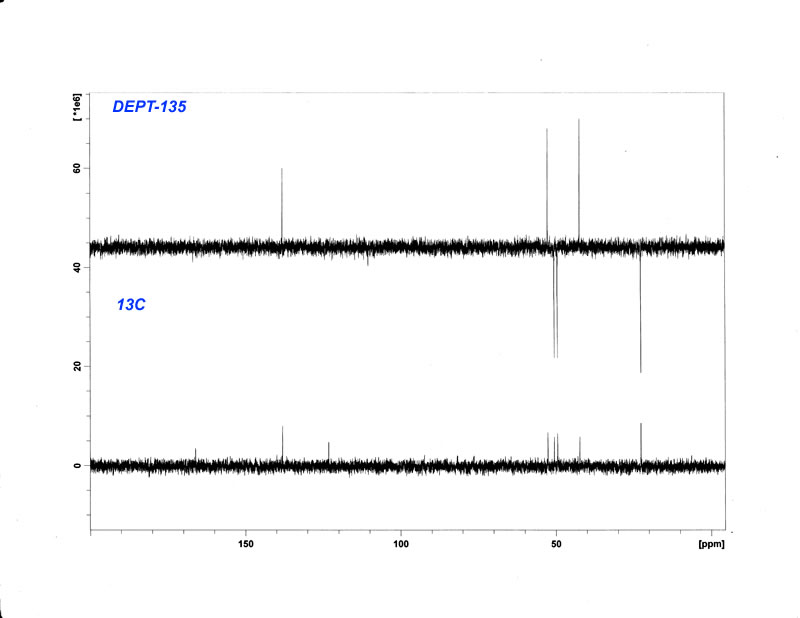
13C-NMR v. DEPT-135 Purified Hydrobromide (D2O)
References
1. Arkivoc 2009 (ix) 45-56.
2. Eur J Med Chem. 2009, 44(12):4848-54.
3. Eur. J. Pharmacol (1987), 134(1), 61-67
4. J. Am. Chem. Soc. (1951) 73, 5614
5. Chin. J. Pharmaceuticals (Zhongguo Yiyao Gongye Zazhi) 2004, 35(5) 265.
6. Khimiko-Farmatsevticheskii Zhurnal, Vol, 10, No, 11, pp. 90–91, November, 1976.
[Edited on 24-8-2010 by Arrhenius]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Beautiful!
The first total synthesis at Sciencemadness! Your contribution is so perfect that I have nothing to add. Well, except that you misspelled "ethyl
nicotinate" everywhere where "methyl nicotinate" should be. 
Though of topic, while we are on topic of arecoline, I might just as well attach the chapter on arecoline analogues and their SAR for those more
medicinal chemistry oriented rather than total synthesis motivated. There are some interesting synthetic approaches described though:
Muscarinic Agonists for the Central Nervous System
Raymond Baker and Angus M . MacLeod
in: Drug Design for Neuroscience (ed. by Alan P. Kodkowski)
Attachment: Muscarinic agonists for the central nervous system.pdf (581kB)
This file has been downloaded 2192 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Haha, thank you Reviewer #1 - I wont' tell you I made the ethyl ester as well. Changes made as noted  . I'm a little bummed with the yield, but that's life. Perhaps dropping this stuff out of ether as its hydrochloride
would be a viable workup, and circumvent possible ester hydrolysis in the way I did it. Hope you enjoy the NMR - sorry it's a bit difficult to read -
I just love having a DRX-500 at my house! . I'm a little bummed with the yield, but that's life. Perhaps dropping this stuff out of ether as its hydrochloride
would be a viable workup, and circumvent possible ester hydrolysis in the way I did it. Hope you enjoy the NMR - sorry it's a bit difficult to read -
I just love having a DRX-500 at my house!
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Really the first one ever ? Now I had to comment as knowing this, coupled to the fact that I find your report impeccable, deserves nothing less than a
very cheerful congratulations ! 
Are Q&A's still a possibility though ?  A very quick one if you dont mind A very quick one if you dont mind
 . .
Is theyre any reasons, besides the chemical environment, that proton HA has such a high chemical shift ? It certainly has been a while since my last
1H NMR analysis but instinctively I would never have thought such a proton would be that deshielded. Though I tend to point towards a possible reason
that it is part of an alpha-beta unsaturated carbonyl system, I am not sure if it is actually the reason.
Hope my question makes sense. Again my compliments !
QD
[Edited on 29-8-2010 by Quantum_Dom]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quantum_Dom: Vinylic protons are typically in the 5-6ppm region. Add to that the deshielding mesomeric effect afforded by the ester group in
conjugation (would put a + charge on the carbon adjacent to that proton) and its reasonable cause for the its appearance at 7.1ppm.
Arrhenius: Nice work mate. Would be nice to see more work like this in the future. And you have a DRX-500 in your house??! That must cost a
fortune, for the upkeep if not for the purchase!
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Quote: Originally posted by DJF90  | Quantum_Dom: Vinylic protons are typically in the 5-6ppm region. Add to that the deshielding mesomeric effect afforded by the ester group in
conjugation (would put a + charge on the carbon adjacent to that proton) and its reasonable cause for the its appearance at 7.1ppm.
|
Eureka. Thanks DJF90 !
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Awesome writeup. I'm jealous that you have an NMR in your house. Would love to have one of those.
One question, in the first esterification, I notice you use an incredibly large amount of H2SO4. What is your reasoning behind that?
[Edited on 8-29-10 by rrkss]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Quote: Originally posted by rrkss  | I'm jealous that you have an NMR in your house. Would love to have one of those.
One question, in the first esterification, I notice you use an incredibly large amount of H2SO4. What is your reasoning behind that?
[Edited on 8-29-10 by rrkss] |
I was only joking, I merely have access to NMR facilities - I don't own one.  As
for the large excess of sulfuric acid, I tried several times with 'catalytic' or 1eq of acid, and found this to give very poor results. I would guess
this is because the substrate is basic, so you're immediately protonating the substrate, and the conjugate acid is not effective in catalyzing the
esterification. Additionally, using a large excess helps to desiccate the reaction, driving it to higher conversion (Le Chatelier principle). As
for the large excess of sulfuric acid, I tried several times with 'catalytic' or 1eq of acid, and found this to give very poor results. I would guess
this is because the substrate is basic, so you're immediately protonating the substrate, and the conjugate acid is not effective in catalyzing the
esterification. Additionally, using a large excess helps to desiccate the reaction, driving it to higher conversion (Le Chatelier principle).
DJF90: I've only got a few ideas for things to come... nothing to spectacular. I'd like to do something collaborative on here if possible - have
several folks work on different steps. I like palladium catalysis, and was thinking about making dibenzylideneacetone, Pd2(dba)3 from Pd metal (Pd
coins are cheap) and attempting benzopyran or benzodipyran synth via Heck rxn.
[Edited on 30-8-2010 by Arrhenius]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Funny to see how many actually believed one of our members has a 500MHz NMR machine at home, but it is possible after all. If you live in a university
campus then you can kind of say that you have one at home.
In my experience, the fastest and higher yielding Fischer esterification to methyl benzoates is by using 150 mol% H2SO4, 1.5-2 mL MeOH per mmol
substrate, and heating the mixture for about 1 h at 100 °C in an autoclave. It always gives excellent, near to quantitative yields, and was used also
on some aminobenzoic acids where the yields were slightly lower though (~90%). Using conventional reflux took days on some difficult benzoic acids,
particularly some hydroxy substituted ones, and the yields were always considerably worse. Not all acids are that difficult to esterify though, for
example, for most aliphatic ones an hour of reflux in methanol is more than enough.
Arrhenius, here is an idea for you given that you found the reduction of the pyridinium interesting. Sodium dithionite could possibly reduce
pyridinium salts, though using it on methyl nicotinate would probably give a mess and precautions to prevent hydrolysis would have to be used.
However, you could try reducing N-benzyl pyridinium bromide (easily made) by refluxing in Na2S2O4/Na2CO3/H2O. I wander where would it stop and if it
would give good selectivity for one end product.
I have to correct myself. I later remembered that Klute reported the synthesis of zingerone two years ago.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Quote: Originally posted by Nicodem  | | ...precautions to prevent hydrolysis would have to be used. However, you could try reducing N-benzyl pyridinium bromide (easily made) by refluxing in
Na2S2O4/Na2CO3/H2O. I wander where would it stop and if it would give good selectivity for one end product. |
I would be very suprised if this was not hydrolyzed rapidly to the aldehyde (e.g. Zinke aldehyde synthesis; in itself interesting). I think STAB is
quite selective as a 2e reducing agent, whereas dithionite would be initiating single electron reduction. Who knows. I'm not very familiar with
dithionite though. On the note of single electron reductions, I'm very intrigued by the use of silicon in dissolving metal reductions.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | Quote: Originally posted by Nicodem  | | ...precautions to prevent hydrolysis would have to be used. However, you could try reducing N-benzyl pyridinium bromide (easily made) by refluxing in
Na2S2O4/Na2CO3/H2O. I wander where would it stop and if it would give good selectivity for one end product. |
I would be very suprised if this was not hydrolyzed rapidly to the aldehyde (e.g. Zinke aldehyde synthesis; in itself interesting). I think STAB is
quite selective as a 2e reducing agent, whereas dithionite would be initiating single electron reduction. Who knows. I'm not very familiar with
dithionite though. On the note of single electron reductions, I'm very intrigued by the use of silicon in dissolving metal reductions.
|
I'm not aware of dithionite use in SET reductions. I always though it is a typical two electron reducing reagent.
Anyway, I did a preliminary experiment on the reduction of 1-benzylpyridinium chloride with Na2S2O4/Na2CO3/H2O and surprisingly the reduction goes all
the way to N-benzylpiperidine!
Experiment:
To 1-benzylpyridinium chloride (415 mg, 2 mmol), Na2S2O4 (1750 mg, 10 mmol) and Na2CO3 (640 mg, 6 mmol) was added water (25 mL). Some gas evolution
was observed during the dissolution and the resulting yellowish clear solution rapidly discoloured to completely colourless. This solution was
refluxed for 5 h (without reaction progress monitoring), cooled and extracted with ethyl acetate (10 mL). The extract showed one major peak on HPLC
and a little impurity on the more polar side. A HPLC run with a bit of toluene added as an internal standard confirmed that no reductive cleavage of
benzylpyridinium to toluene occurred. TLC also showed one strong spot and some minor impurities. Part of the extract was rotavaped and analysed by
NMR, which confirmed that the major product is N-benzylpiperidine (see Org. Synth. for 1H and 13C NMR spectral data of benzylpiperidine). NMR fits well and all the DEPT135 peaks are present also in the cited
13C NMR. The IR spectra also matched.
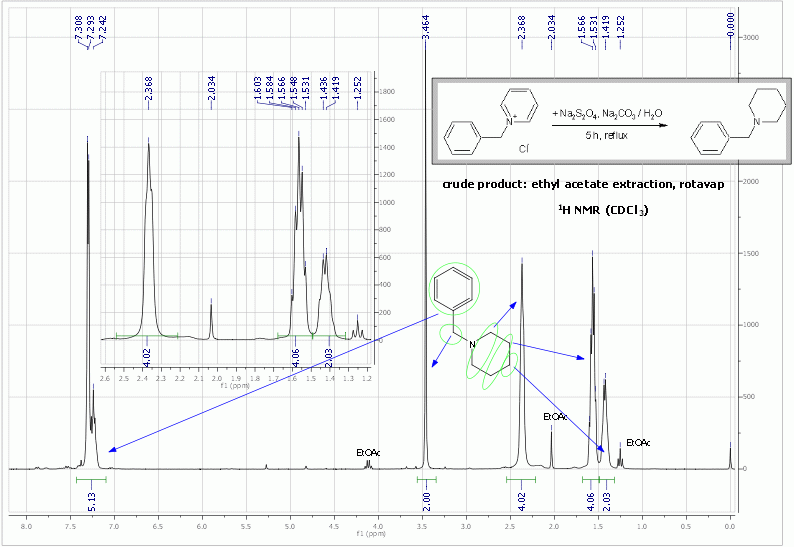
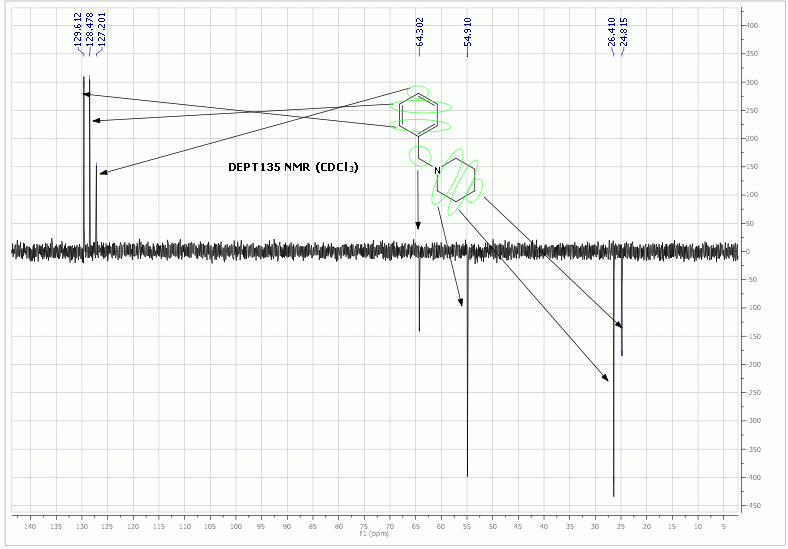
Edit: I corrected the phase in the DEPT135 spectra, which was previously upside down, and reuploaded it. For those who don't know what the peak phase
in DEPT means: CH&CH3 carbons give a + phase (up), while all CH2 give a - phase (down), quaternary carbons give no signal (DEPT spectra are based
on 13C-1H coupling), chemical shifts remain identical to 13C NMR.
[Edited on 10/9/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Interesting. I didn't search very thoroughly, but it seems dithionite is known for reducing pyridinium salts, as well as an array of other
functionality. As for the mechanism, I figured it was single electron reduction on the basis of dithionite generating alkyl radicals from alkyl
halides. I don't know if it's still the consensus, but a couple papers implicate SET.
See:
J. Org. Chem. (1981) 46, 5457
J. Am. Chem. Soc. (1955) 77, 2261
I'd love to see your mechanistic proposal though 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Ah, damn, I should have searched the literature first! I found it such a coincidence that I found a vial of benzylpyridinium chloride just a couple
days after my previous reply above, that I just had to set up a reduction experiment. Now that I searched I found plenty of papers on the reduction of
pyridiniums by Na2S2O4, though nearly all describing the reduction to 1,4-dihydropyridines. However, in an Chemistry Letters paper they obtained complete reductions on some substrates and say: "When N-benzylpyridinium chloride was reduced under the same
conditions, the product isolated was N-benzylpiperidine (37%)."
As far as the mechanism goes, I always thought it goes kind of like in the path B of the JOC paper you cited above. Though the results of that study
mainly confirms it, some other papers I encountered searching raised some new doubts. So I got convinced things may not be that simple. It is
surprising how easily single electron transfer occurs in some of these sulfo reagents(considering in particular the related thiourea dioxide), so SET reductions are not to be ruled out.
Well, I learn new things every day. Bellow is the relevant excerpt from Comprehensive organic synthesis: Reduction, Volume 8
(Ian Fleming) that kind of minireviews this (off) topic:
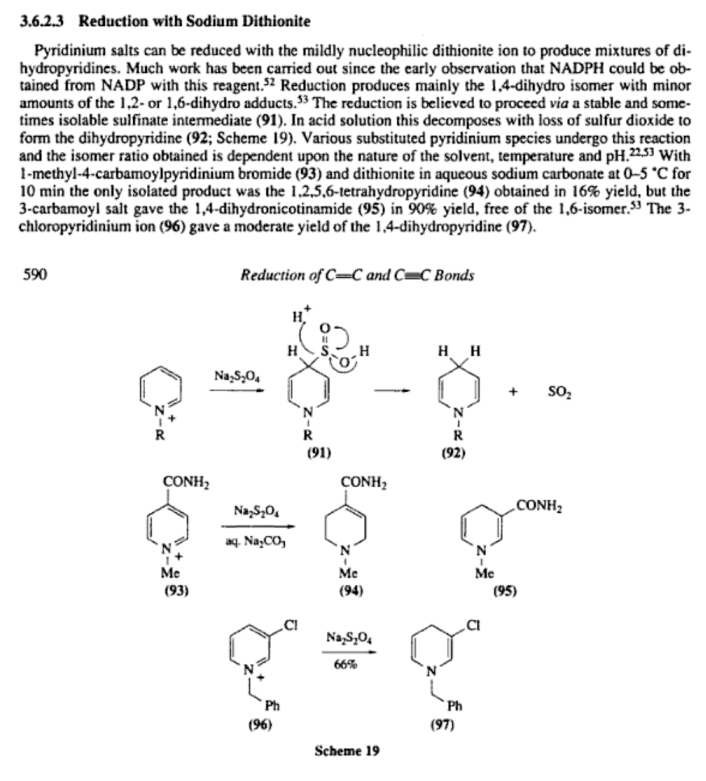
[Edited on 5/9/2010 by Nicodem]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ya, I think the dihydropyridine outcome sort of points to single electron processes. I can see how substitution might greatly alter this Birch-type
reactivity. Hm. Very interesting. It's great that the reaction was pretty clean for you. No benzylamine either it sounds like - so no hydrolysis of
intermediates. The obvious difference is in the conditions. It's plausible that the dihydropyridine (shown above) is rapidly formed (they say
0-10ºC, 10min), and forced to saturation under refluxing conditions, which is not possible under Birch conditions.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Nice work there Arrhenius!   Btw, have you tried using plain NaBH4 for the reduction of pyridinium for a better yield? During model studies, a
couple years ago, I got decent yields of arecoline and some of its analogues from corresponding pyridiniums, using plain NaBH4. I have the
lab-notebook at my previous job so I can't give any experimental details, but I followed the method in last entry (lysergic acid): https://www.erowid.org/archive/rhodium/chemistry/lysergic.he... Btw, have you tried using plain NaBH4 for the reduction of pyridinium for a better yield? During model studies, a
couple years ago, I got decent yields of arecoline and some of its analogues from corresponding pyridiniums, using plain NaBH4. I have the
lab-notebook at my previous job so I can't give any experimental details, but I followed the method in last entry (lysergic acid): https://www.erowid.org/archive/rhodium/chemistry/lysergic.he...
[Edited on 6-9-2010 by Sandmeyer]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Thank you. No, I did not try plain borohydride. As I mentioned above, the literature cites STAB as being a milder more selective reagent. NaBH4 in
alcohol seems like it would work fine. I'm not really sure why some literature syntheses use biphasic reduction conditions - seems unnecessary and
likely to degrade the pyridinium salt.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
There is a nice, scaled-up procedure from a paper entitled Tetrahydropyridyloxadiazoles: semi-rigid muscarinic ligands, see J. Med. Chem., 1991, 34
(3), pp 1086–1094 DOI: 10.1021/jm00107a032
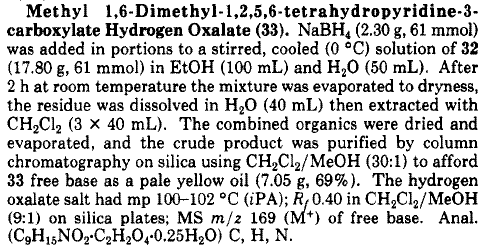
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ah. I'm aware that this works, but I was concerned about over reduced side products, which might be difficult to remove by means other than
chromatography. Along that line, I'm willing to bet that chromatography or distillation post-reduction would significantly improve yield.
Chromatography free synthesis is commendable, but my report above definitely didn't pull it off that well.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Arrhenius  | | Ah. I'm aware that this works, but I was concerned about over reduced side products, which might be difficult to remove by means other than
chromatography. Along that line, I'm willing to bet that chromatography or distillation post-reduction would significantly improve yield.
Chromatography free synthesis is commendable, but my report above definitely didn't pull it off that well. |
Real men use chromatography. Honestly, having done a fair share of them now, it's not all that difficult for small quantities (of course, I'm from the
flash chromatography camp and we had solvent recycling stills available)
If I'd written up my total synthesis of 6,6'-dibromoindigo a few months ago, I would have beaten you to first total synth on science madness. But oh
well, I'll enjoy my tube of precious purple powder. 
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
It might be worthwhile to try aluminum amalgam for reducing the methyl nicotinate methiodide, since it is also a mild agent capable of reducing
iminiums.
|
|
|