| Pages:
1
2 |
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH, PHILOU know exactly what i'm talking about....
back to the thread here, any unreferenced information has no scientific value to me...it will only amuse me...
Dany.
|
|
|
Davin
Harmless

Posts: 36
Registered: 5-12-2012
Member Is Offline
Mood: No Mood
|
|
This is simply unreferenced speculation (Dany  )...but I would wonder if the
hexanitro may be more interesting than the dodecanitro....neither will be completely planar as a result of C-H and N-O or N-O and N-O crowding, but
due to the potential formation of C-H...O which should be a more favorable interaction than two nitro group oxygens repelling each other,
the dodecanitro may have a torsion angle of the nitros relative to the ring that is further from planar than in the hexanitro. But if your synthesis
methodology works, it would be easy enough to try both flavours. )...but I would wonder if the
hexanitro may be more interesting than the dodecanitro....neither will be completely planar as a result of C-H and N-O or N-O and N-O crowding, but
due to the potential formation of C-H...O which should be a more favorable interaction than two nitro group oxygens repelling each other,
the dodecanitro may have a torsion angle of the nitros relative to the ring that is further from planar than in the hexanitro. But if your synthesis
methodology works, it would be easy enough to try both flavours.
I am without a mass spec currently, but may give it a shot eventually when my plate is far less full.
[Edited on 23-4-2014 by Davin]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I was so busy writting replies to posts that I didn't noticed it  . Thank you
DeltaH, you made my day! . Thank you
DeltaH, you made my day!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Dany  | deltaH, PHILOU know exactly what i'm talking about....
back to the thread here, any unreferenced information has no scientific value to me...it will only amuse me...
Dany. |
No fight! There is room in this forum for everybody to post ones idea, refexion, criticism and theorems  . .
Indeed I know exactly what Dany refers to. I'm working on the writing to make it understandable. The concept is to explain the all process that lead
to the theorical concept and the conclusion that ended to a theorem.
I think that the process is even so important than the theorem.
At first I thought I would make the writing by step by step postings...but replies from other posters would interfere with the general overview...so I
prefer to put it in a single post (if it succeeds  ). ).
"any unreferenced information has no scientific value to me...it will only amuse me... " "
Amusement/Fun is a good first step; but how do you do when there is no reference yet  and only reproductible experiments and only reproductible experiments  ...then I guess you won't accept
autoreference because it would be intrinsically partial and biased science proof ...then I guess you won't accept
autoreference because it would be intrinsically partial and biased science proof  (Just kidding). (Just kidding).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | | I second that Dany, PHILOU that really needs thread dedicated to it! I have another musing concerning quinone that I
will also post soon, I think there is much scope for EM and quinone and perhaps a little overlooked. |
Every idea or group of ideas would need specific tread...this forum offers enough room for that. 
As explained to DAR:
You have certainly noticed that the more you know about chemistry of Energetic materials, the more you get ideas in an exponential fashion from a
single idea...most of those ideas are not investigated yet because the field is so vast.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Davin  |
This is simply unreferenced speculation (Dany  )...but I would wonder if the
hexanitro may be more interesting than the dodecanitro....neither will be completely planar as a result of C-H and N-O or N-O and N-O crowding, but
due to the potential formation of C-H...O which should be a more favorable interaction than two nitro group oxygens repelling each other,
the dodecanitro may have a torsion angle of the nitros relative to the ring that is further from planar than in the hexanitro. But if your synthesis
methodology works, it would be easy enough to try both flavours. )...but I would wonder if the
hexanitro may be more interesting than the dodecanitro....neither will be completely planar as a result of C-H and N-O or N-O and N-O crowding, but
due to the potential formation of C-H...O which should be a more favorable interaction than two nitro group oxygens repelling each other,
the dodecanitro may have a torsion angle of the nitros relative to the ring that is further from planar than in the hexanitro. But if your synthesis
methodology works, it would be easy enough to try both flavours.
I am without a mass spec currently, but may give it a shot eventually when my plate is far less full.
[Edited on 23-4-2014 by Davin] |
In the original pernitro-radialene tread from Franklyn (nitrocarbon), I mentionned:
"Also radialene must be quite unstable and prompt to rearrange into a central aromatic ring and 3 cyclobutane rings between C1 and C2, C3 and C4 and
between C5 and C6.
The very same may happen with our putative dodecanitroradialene??"
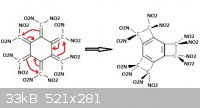
This would preclude any inter-nitro steric hindrance.
[Edited on 23-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
2 |