| Pages:
1
..
8
9
10
11
12
..
77 |
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Iron(II) sulfate heptahydrate
Been recrystallizing 1 kg of this compound and got nice crystals.. for more click here IRON SULPHATE CRYSTALS
<img src="http://chem.pieceofscience.com/wp-content/uploads/2014/03/2.jpg" width="900" height="597">
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
How did you do this? I tried making crystals from iron II sulfate and the solution turned yellow. Im guessing from dissolved oxygen.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Beautiful, Hegi, just beautiful! I tried making iron sulfate through replacement of copper sulfate and my crystals turned out a more greenish color
rather than this aqua color.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|



After long-long time one of my compounds crystallized out. Sadly according to the recorded NMR spectra we are still not able to tell what is in the
flask, but it still looks great.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by Brain&Force  | | Beautiful, Hegi, just beautiful! I tried making iron sulfate through replacement of copper sulfate and my crystals turned out a more greenish color
rather than this aqua color. |
try to dissolve it in near boiling distilled water and add few milliliters of concentrated sulphuric acid, then let it overnight in fridge and you
should be able to get that color. I´v been surprised too cause whenever I saw it before it was oxidized or rather hydrolyzed. 
Kristof - what is it suposed to be? ..
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Quote: Originally posted by Hegi  | Quote: Originally posted by NeonPulse  | Re-crystallizing some Trinitrophenol. Got a nice crop of well formed needle shaped crystals in the beaker....
 i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics. i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics.
|
What solvent did you use? ... I prepared today few grams of TNP and then dissolved it in warm ethanol.. so now I´m waiting for crystals..
|
I only dissolved the raw TNP in boiling distilled water to get a super saturated solution and very slow cooling the crystals grow as it cools. no
solvent needed here. there are few helpful threads in the EM forum on this.
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|




My sample of bismuth. Love that metal.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Made from this? https://www.youtube.com/watch?v=uRyPG6iJyjI
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
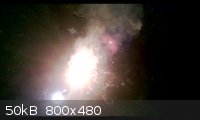
Reaction between ~3gm pyrotechnical grade Aluminum powder, and ~8-10 drops of Mg2O7.
Reaction delay: ~22 seconds
I put a series of pictures taken within 5 seconds. (Excluding first and last, to show start/end)
 
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
magnesium heptoxide?
I doubt it
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Why do you doubt it? I'm not the best at videos, so I went for screenshots of the video, but just for you, I'll upload the video - which should clear
any doubts.
http://youtu.be/h4AuUNRXo-o
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
'cause maybe you meant manganese heptoxide? Mn2O7?
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
oopsies... Your right. My bad. I feel silly, Mg and Mn:\
[Edited on 4-7-2014 by numos]
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
I thought I'd try some Kristof/Hegi style photography... came out ok haha. Here is some Benzoic acid that i made by oxidising toluene via KMnO4

[Edited on 7-4-2014 by HeYBrO]
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by HeYBrO  | I thought I'd try some Kristof/Hegi style photography... came out ok haha. Here is some Benzoic acid that i made by oxidising toluene via KMnO4
|
nice try bro... I love that oxidation... fast and cheap way to produce your own benzoic acid..

Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by Hegi  |
nice try bro... I love that oxidation... fast and cheap way to produce your own benzoic acid..
|
Thanks. I was going to edit the contrast and sharpness but i though against it. Looks much better, cheers. 
[Edited on 7-4-2014 by HeYBrO]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


Something special crystallized at the bottom of a flask, a cuneane based compound, what was obtained by the rearrangement of a cubane based molecule.
What is cubane and what is cuneane and what is this rearrangement? Cubane (1) is a square shaped and cuneane (2) is an wedge shaped molecule, both are
strained.

For more information read this recently published article what was published by my supervisor:
Solvent-induced, selective rearrangement of hydrogen cubane-1,4-dicarboxylate to hydrogen cuneane-2,6-dicarboxylate
Gábor Durkó, István Jalsovszky
Tetrahedron, Volume 69, Issue 25, Pages 5160–5163
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Aw, come on! Nobody's going to top the brothers Hegedus!
I'm just wondering, kristofvagyok, what license, if any, do your photos fall under?
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Kristof, so epic, the picture and the molecule as well... high level organic chemistry.
Freshly prepared Mohr´s salt.

Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Here is my first bromine isolation! I distilled it today from the reaction of NaBr+KMnO4+HCl. It has some water in it, but I don't really
mind all that much, and I think it still looks really cool.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Nice! I've been isolating bromine several times this week, to find the cheapest method. Are you sealing it in an ampule?
[EDIT] Or is it in an ampule already? It's hard to see what kind of container it's in.
[Edited on 13-4-2014 by Zyklonb]
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Unfortunately, it's not in an ampule. It's just a normal vial with a teflon lined lid. It should keep it in, although I really wish I could have
ampuled it.
|
|
|
Godspeed429
Harmless

Posts: 11
Registered: 24-3-2013
Member Is Offline
Mood: +2
|
|
52 gram crystal of Aluminum potassium sulfate.

|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Some recrystallized KClO3, cooled very slowly overnight in a beaker...wrapped in a towel and then placed in a thermos lunch box. Some crystals are
hard to capture on camera but a few plates are the size of dimes. Beautiful 

|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Attribution Non-commercial Share Alike (by-nc-sa)
And another picture:

Trying out a new UV lamp.
The mercury vapor lamp is placed in a double walled quartz tube what will serve as a water cooled, tempered UV light source, used for photochemistry.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
| Pages:
1
..
8
9
10
11
12
..
77 |