predoc14
Harmless

Posts: 3
Registered: 1-4-2014
Member Is Offline
Mood: No Mood
|
|
LSD-25 to 2-Bromolysergic acid
Hello everyone,
I'm working on a paper for the synthesis of 2-bromo-LSD from LSD-25 and was wondering if someone could help me propose a synthesis for attaching
bromine. 2-Bromo-LSD is the non-hallucinogenic form of LSD that has some medicinal use in treating cluster headaches.
The synthesis of 2-bromolysergic acid diethylamide has been described by Troxler and Hofmann (HeIv. Chim. Acta 40:2160 (1957)) but it appears to be in
German. Does anyone have an idea where I can find synthesis information for 2-bromo-LSD?
LSD-25

2-Bromo-LSD
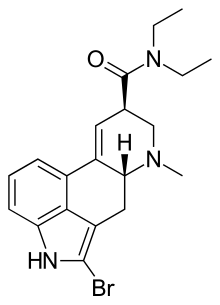
Thanks so much!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by predoc14  | | The synthesis of 2-bromolysergic acid diethylamide has been described by Troxler and Hofmann (HeIv. Chim. Acta 40:2160 (1957)) but it appears to be in
German. |
Just what makes you think that articles written in German are not good enough?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
He's new, and probably doesn't know how to get a translation.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I skimmed, but it looks like maybe J. Med. Chem., Vol. 16, No. 5 (1973) has relevance. Check table V for structure, and the next section has
experimentals.
|
|
|
predoc14
Harmless

Posts: 3
Registered: 1-4-2014
Member Is Offline
Mood: No Mood
|
|
Where can I get a free translation?
I ordered this article in German and should have it by the end of the week. Substitutionen am Ringsystem der Lysergsäure. III. Halogenierung. 45.
Mitteilung über Mutterkornalkaloide (pages 2160–2170)
F. Troxler and A. Hofmann
Article first published online: 24 OCT 2004 | DOI: 10.1002/hlca.19570400716
What is the best way to get this translated for free? There has to be an English translated synthesis out there.
Thanks!
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Google translate is a good place to start. It'll give you the broad strokes, and it's not bad with chemical stuff, either - although copy-pasting from
old pdf's will require some fixing up!
This dictionary is also helpful:
http://www-sul.stanford.edu/depts/swain/beilstein/bedict1.ht...
Doing this over and over is how I've gained my rudimentary understanding of German chemical writing - I doubt I could intelligibly speak a word of it,
but I can usually manage to read a procedure now.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Sometimes members offer to translate on this forum. I've actually had pretty good luck using various free internet translation services, a German
dictionary, and analogous reactions.
Speaking of analogous reactions, was my citation of any use? It appears to have the preparation of the isopropyl and n-butyl secondary amide
derivatives via bromination with NBS, and is in English.
|
|
|
predoc14
Harmless

Posts: 3
Registered: 1-4-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  | Sometimes members offer to translate on this forum. I've actually had pretty good luck using various free internet translation services, a German
dictionary, and analogous reactions.
Speaking of analogous reactions, was my citation of any use? It appears to have the preparation of the isopropyl and n-butyl secondary amide
derivatives via bromination with NBS, and is in English. |
I searched through volume 16 issue 5 and was unable to find the article you referenced. Do you remember the title?
Any ideas anyone on the synthesis of 2-bromo-LSD from LSD?
Thanks
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by predoc14  |
I searched through volume 16 issue 5 and was unable to find the article you referenced. Do you remember the title?
Any ideas anyone on the synthesis of 2-bromo-LSD from LSD?
Thanks |
Emetic activity of reduced lysergamides
Fatima N. Johnson, Istvan E. Ary, David G. Teiger, Ronald J. Kassel
pp 532–537
Publication Date: May 1, 1973 (Article)
DOI: 10.1021/jm00263a028
Pretty sure this is exactly the kind of thing you want.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Here is the important part of that paper. It looks pretty simple, just dissolve in dioxanes, add a slight excess of NBS and heat for a short time.
That sound pretty straightforward. Just don't lick your spatula weighing the SM.

|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
And don't get caught with the starting material.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
*FWOOSH*
Hazard to Self
 
Posts: 90
Registered: 6-9-2013
Location: ooo esss ahhh
Member Is Offline
Mood: manic
|
|
Shoot, but I always lick my spatula!
This is interesting, I'd never really looked into the medicinal potential of LSD derivatives. It makes sense though, look at whats been done with
tryptamines! (I realize LSD is technically a tryptamine, no smart asses please...)
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Where exactly he got the starting material from is the question! XD
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
How about starting from bromocriptine?
It already has the Br and is not difficult to get.
wikipedia.org/wiki/Bromocriptine
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gregxy  | How about starting from bromocriptine?
It already has the Br and is not difficult to get.
wikipedia.org/wiki/Bromocriptine
|
Looked like Garbrecht's 1958 paper (J. Org. Chem. 24, 368-372 (1958)) applied to bromocriptine would be a good proposal for the paper. Not sure
exactly what you'd do about the halogen during the animations, but not going to spend much effort considering it. Definitely a reason bromocriptine
uses the same reaction I mentioned above to brominate last. Much less paperwork going a bromocriptine/ergocriptine route if ever applied practically
in a legitimate med chem setting, I would imagine. Speaking of, I wonder what the OP's paper is for, exactly.
[Edited on 3-4-2014 by Chemosynthesis]
|
|
|