cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Electrolytic Cell Question
Hello!
I've been a lurker here for a while, and just joined.
I have a question about a simple electrolytic cell that I set up.
I was trying to electrolyse vinegar in order to produce Cu(CH3COO)2 for crystal growing. I know that it is really easy to
produce by dissolving Cu metal in vinegar. However, that takes forever, and I wanted a faster way to make it. If I had glacial acetic acid, I would
have used that, but because I don't really have many reagents on hand at home. (Due to this fact, and the fact that I love to learn, I tend to produce
any reagents I need. Granted, I have not really done any complex experiments up to this point, but I hope to do so in the future. I know that a
homemade reagent will often be impure, but it is still a fun learning experience.)
So, I assembled a simple cell. I used Cu wire for both the anode and the cathode, and used a 9V battery as the power source. I used plain, white
distilled vinegar from the store.
I turned on the current, and bubbles appeared at both electrodes. I expected H2 and O2 to be produced, so it appeared to be
going well. An hour later, a faint blue was diffusing through the solution. I took this as a good sign, as Cu++ ions produce a nice blue
solution.
I let the reaction run overnight, as the amounts of H2 and O2 shouldn't have been significant.
When I checked on it in the morning, the solution was a nice blue, much like a CuSO4 solution would be. I removed the electrodes and
covered the container with a paper towel to keep contaminants out.
Suddenly, two days later, the solution turned brown. This threw me off, as no percipitate formed, and I don't know of any soluble Cu salts that result
in a brown solution. I let the solution evaporate down to dryness over the course of about a month. This resulted in a few small (max 2mm)
Cu(CH3COO)2 crystals, and an orangish, fine crust. I added distilled water to this mix, and found that the orangish crust,
without any visible crystals, dissolved almost instantly. I then decanted the solution of the copper II acetate crystals, allowing me to seperate the
two solutions.
Now, I've asked the chemistry teacher at my high school what this could be, but she has no idea. She thought that maybe the Cu had oxidized, but I
don't think that happened as neither copper I oxide nor copper II oxide are soluble.
What could this brownish solution be?
NOTE: Since I tried the electrolysis, I've successfully dissolved Cu wire in vinegar, so I have the copper II acetate for crystal growing.
Sorry for such a long first post, but there is not really a way to write it more concisely!
Thanks!
-cpman
[Edited on 12-10-2013 by cpman]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you want to make copper acetate, you're better off making basic copper carbonate from copper sulfate and sodium carbonate, then reacting it with
vinegar.
The brown stuff sounds to me like it could be an iron compound, formed from impurities in the copper or possibly from your electrolytic cell,
depending on what it's made from. Also, it could possibly be some impurity of the vinegar.
Can you provide pictures, and also try testing for iron?
Too bad you live in Texas, where it's illegal to own even an Erlenmeyer flask . .
[Edited on 10-12-2013 by Cheddite Cheese]
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
I imagine it is from a vinegar impurity. The cell was just a plastic cup with around 200ml of vinegar. The Cu wire was the only metal that touched the
solution. Adding ammonia or NaOH would allow me to test for iron ions, right?
The only problem is the solution likely still has copper ions in it, which may make the results harder to decipher. Fortunately I've seen
Cu(OH)2 before, which would allow me to tell it apart from the iron hydroxide complexes that would percipitate out due to ammonia or NaOH.
I think I should use ammonia, as that is easier for me to get, and the copper hydroxide will dissolve in it.
I'm fairly certain that the Cu wire is pure, as I use the same source to make clean, pure copper II acetate with vinegar, and there were not any
problems with that.
[Edited on 12-10-2013 by cpman]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cpman  | | I'm fairly certain that the Cu wire is pure, as I use the same source to make clean, pure copper II acetate with vinegar, and there were not any
problems with that. |
How did you determine the purity of your copper(II) acetate?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Copper wire is usually quite pure, since small amounts of impurities will dramatically decrease the conductivity- when I took analytical chemistry,
electrical wire was a primary standard for copper. I'd hazard a guess that it was some organic crud from the vinegar or cup.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Could you try decomposing it with heat (i.e. burning it) to see if it is organic?
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Well, all that came out of the solution when I evaporated this vinegar was copper II acetate crystals. Looking at it under my dissecting microscope at
30x, I could not see anything but crystals of the copper II acetate. Also, crystallization is a very amazing purification method, so the fact that the
crystals have been washed with distilled H2O likely means the only thing they contain is copper II acetate, with some water and acetic acid
molecules incorporated as the solvent of crystallization. I know this is not the best method to verify purity, but they were certainly much more pure
than those produced by electrolysis.
I probably will not do this until I get some ammonia to test it for Fe.
Thanks for all the helpful suggestions!
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
I just used some KOH I extracted from a PH lowering solution, and got a gelatinous, reddish-brown percipitate. This meets the description of the
percipitate formed by Fe ions using NaOH, which should have a similar effect to KOH...
Thanks Cheddite Cheese for the idea of Fe ions!
|
|
|
jwpa17
Harmless

Posts: 45
Registered: 28-5-2013
Member Is Offline
Mood: No Mood
|
|
I tend to agree with DragonicAcid. Copper wire is usually pretty pure. And I'm a bit curious about your system - it sounds as if your anode and
cathode were separated in space, but no diffusion barrier to prevent the products from mixing. It seems pretty clear that you oxidized the copper to
make copper II ion (the blue color). But I can't imagine what was reduced. I suppose it was the water, giving molecular hydrogen and hydroxide,
which would then reaction with the acetic acid to give acetate?
You let the solution sit two days, and then got brown. You evaporated the solution and got some blue crystals (which may or may not have been copper
acetate) and an orange "crust." You add cold water and the orange dissolves, but the blue crystals don't. Except acetates are soluble in water. And
most orange iron compounds are iron III, which isn't. And a lot of metal hydroxides are "gelatinous" precipitates. (Although copper hydroxide is
blue, not orange.
And one more thing - an amp a day is about a mole. You used a 9 V battery overnight. Assuming that's about a 400 mA-hr capacity, you can only
produce about 10-20 mmol of electrons, or maybe 5 mmol of copper II ions. That gives a nearly a gram of product. And the Wikipedia entry for copper
acetate describes the crystals as dark green, not blue. Of course, that's for anhydrous, not hydrated, which is what I'd expect you'd have made.
So I can't explain what you observed, but I'm not convinced the iron explanation is correct. Can you give us more details about your cell and
process? From your description above, I can't see where the iron came from.
Very interesting.
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
I believe the iron came from the vinegar.
The blue crystals were a really dark color, about the same formed by dissolving Cu in vinegar. They did dissolve eventually, but took much longer than
the orangish crust. Because of that, I decanted the brownish stuff off of the crystals to begin seperating the two.
There was no diffusion barrier. The bubbles coming from the cathode. This was presumably hydrogen from either the water or the acetic acid in the
vinegar. Theoretically, some playing could have occurred, though I would not be able to tell as it would be Cu on Cu.
The acetic acid partially dissociates in water to H+ and CH3COO- ions, which lead me to believe that at least some
copper II acetate would be formed. Presumably, some of the non-dissociated acetic acid would be seperate a by electrolysis. Considering the fact that
I saw no Cu(OH)2 percipitate at the cathode, any Cu(OH)2 formed must have reacted with the acetic acid. When a similar cell was
done with a sodium bicarbonate electrolyte, copious amounts of Cu(OH)2 and CuCO3 were formed at the cathode, which led me to
believe that some Cu(OH)2 would have formed.
In the past, I'd found that Cu(OH)2 can be dissolved in vinegar to make copper acetate. Knowing this, I presumed that any water that got
reduced would solely help te production of copper acetate by this method.
The cell was really simple, two copper wires clipped to opposite sides of a plastic cup with alligator clip wires. The other ends of these wires were
attached to a 9V battery. Undiluted store bought white vinegar filled the cup.
[Edited on 12-14-2013 by cpman]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by cpman  | | The cell was really simple, two copper wires clipped to opposite sides of a plastic cup with alligator clip wires. The other ends of these wires were
attached to a 9V battery. Undiluted store bought white vinegar filled the cup. |
Were those alligator clips touching the solution at all?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
jwpa17
Harmless

Posts: 45
Registered: 28-5-2013
Member Is Offline
Mood: No Mood
|
|
cpman - there's no question but that you made copper II ions, and when you evaporated the solution, you got copper II acetate. Your description of
the process makes sense, and you wouldn't see copper hydroxide precipitate because there's very little hydroxide, except perhaps very local to the
cathode. Any hydroxide will react with the acid to make water and acetate.
DraconicAcid's onto something with the alligator clips. If you still know which you used, check them carefully for corrosion - look for tarnished
areas.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cpman  | I just used some KOH I extracted from a PH lowering solution, and got a gelatinous, reddish-brown percipitate. This meets the description of the
percipitate formed by Fe ions using NaOH, which should have a similar effect to KOH...
Thanks Cheddite Cheese for the idea of Fe ions! |
Ph lowering solution would acidify the solution. Basic pH is above 7 not below it.
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
I know that KOH raises PH.
That was just a typo...
Also, I just checked and found a small amount of corrosion on one of the alligator clips.
That would help explain the iron ions in solution...
|
|
|
jwpa17
Harmless

Posts: 45
Registered: 28-5-2013
Member Is Offline
Mood: No Mood
|
|
If you want to repeat the experiment, try supporting the copper electrodes by taping them to a support (a popsicle stick? a bit of dowel) laying
across the top of your container, leaving a centimeter or so extending above the support, and then clip the leads to that extension. That way,
there's virtually no possibility of the clip getting into the electrolyte solution.
Also, a 9V battery seems a bit like overkill - the oxidation potential of copper is only about 0.4 V. That doesn't consider any overpotentials, but
still... From what Wikipedia says, an alkaline D-cell has a current capacity about 20x that of a 9V battery, so you'd quite probably be better off
with 2 D-cells in series. Might be worthwhile to measure the voltage drop or current, if you have the capability.
Good luck.
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
aqueous ammonia easily separates copper salts (which dissolve) from iron (which does not), so i advise to use that.
[Edited on 2013-12-15 by ElectroWin]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A galvanic cell approach. See my prior comments and source link on SM with respect to metal-air batteries. With respect to the current application,
dilute H2O2 is the oxygen source.
What I just performed, added a Copper source (new pennies) to vinegar, a small amount of salt (some discussion in the literature suggests that a
better electrolyte is actually sea salt, which I employed here) and then dilute H2O2. Microwave for 30 seconds to start the reaction at which point an
obvious reaction ensures. Within five minutes, a characteristic aqua blue color.
The electrochemistry includes the formation of Cu(OH)2. Side reactions are the action of the dilute acetic acid on the copper hydroxide creatng copper
acetate and the loss of some of the H2O2 on contact with CuO liberating oxygen. The latter O2 is still available, however, in a closed vessel for
consumption.
My take on the electrochemistry is the anode oxidation half-reaction:
2 Cu + 4 OH− → 2 Cu(OH)2 + 4e−
The cathode reduction half-reaction:
2 H2O2 + 4e− → 4 OH−
The net electrochemical reaction would then be:
2 Cu + 2 H2O2 → 2 Cu(OH)2
Some of the standard chemical side reactions of interest:
Cu(OH)2 + 2 HOAc → Cu(OAc)2 + 2 H2O
2 H2O2 --CuO→ 2 H2O + O2
Note, there is no need to measure as one can assume when using dilute H2O2 that the Copper is in excess and will require further additions of H2O2
(evident when the bubbling ceases).
Advantages of this approach is that it is relatively quick using homehold chemicals without the need for prolonged heating. A drawback is using too
much salt as the electrolyte could introduce significant sodium and chloride contamination of the copper acetate product. However, using too little or
no salt, and the reaction proceeds much more slowly.
[Edit] Here are two pictures. The first is a few minutes after a 30 second microwave warm-up, and the last 10 minutes latter already showing
distinctive coloring. Note, one can use a large number of pennies and remove them when their surface is cleaned. Otherwise, with time the copper plate
could be pierced exposing the underlying Zinc core and the chemistry accordingly changes.
 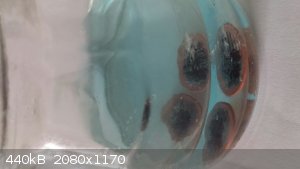
[Edited on 19-10-2014 by AJKOER]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Found this patent in my searches and this seems like a good thread to post it in. Very interesting read.
Chemical synthesis with electric precipitation: United States Patent 2279583
"My invention relates broadly to chemical synthesis in the gaseous or vapor stage and more particularly to an electrode arrangement for facilitating
the chemical reaction and producing electric precipitation of the products of reaction."
Attachment: US2279583A.pdf (317kB)
This file has been downloaded 456 times
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|