spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
3,4-(methylenedioxy)propiophenone
Hello all,
I have an ongoing research interest in this compound and I understand that it may also be of interest to many people whose motivations I will not
speculate on.
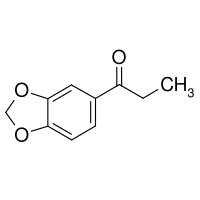
A supposedly effective (and relatively direct) synthetic route to this compound can be found on page 12 of international patent WO 96/39133 involving the Iodine catalysed acylation of 1,3-Benzodioxole with Propanoic Anhydride and subsequent extraction
from the reaction matrix by distillation under high vacuum. The procedure in the patent appears to have itself been based on a 1954 publication from
the Journal of the American Chemical Society (J. Am. Chem. Soc., 1954, 76 (20), pp 5150–5150) and what is notable is that the violet coloured vapour of their reaction mixtures disappeared
under reflux in successful acylations.
A series of reactions were recently attempted and took place by the general procedure outlined below:
To a clean, dry round-bottomed flask was added 28.8g of 1,3-Benzodioxole and 35.4g of Propanoic Anhydride followed by 1.2g of elemental Iodine which
dissolved rapidly with magnetic stirring to afford a dark red-to-purple solution. The reaction mixture was brought to reflux with visible condensate
at a bath temperature of around 116°c initially affording a vapour and condensate of the same dark red-to-purple colour of the reaction mixture.
Reflux conditions were maintained for 3.5 hours with a rise in bath temperature to around 135°c over the period and a distinct colour change noted
after around 3 hours affording at first a pale brown condensate which rapidly turned clear with the reaction mixture appearing dark brown-to-black
with a significant quantity of similarly-coloured precipitate forming and depositing on the sides of the flask.
A workup was attempted in accordance with the procedure outlined in the aforementioned patent and afforded the expected volatile fractions and a
subsequent clear fraction of 1,3-Benzodioxole under the expected conditions leaving a thick black oil which failed to yield a distillate at conditions
even in excess of those outlined in the patent procedure. After being allowed to cool to room temperature, the thick black oil took on the properties
of a glassy solid which appeared to be somewhat water soluble. This solid was broken up and stirred under 200ml of DCM and 200ml of distilled water
and the organic fraction separated and washed with dilute Sodium Bisulfite solution. The DCM was removed by heating to afford an immeasurably small
(<0.1g) quantity of a light brown liquid that quickly solidified on removal from heat and may be speculated to be a small quantity of the desired
product of the reaction.
Following the obvious failure of this procedure the reaction was repeated with fresh 1,3-Benzodioxole and a modified workup was used based on the
procedure outlined in U.S. Patent 6,342,613. In this workup the previous resulting reaction mixture was allowed to cool and 100ml of DCM and 100ml of distilled water
was added and allowed to stir for 30 minutes affording two black layers containing a suspension of rubbery black solids which were removed by
filtration and the liquids added to a separatory funnel and separated (requiring the use of a laser pointer to determine the interface between the two
layers due to the similarity in colour). Under direct light the dark brown-to-black organic layer appeared deep red in colour. The organic layer was
washed successively with dilute Sodium Bicarbonate solution until cessation of bubbling, followed by dilute Sodium Bisulfite solution followed by a
final rinse of water with each wash causing a notable lightening of the organic layer. The organic layer was then transferred to a round bottomed
flask and the DCM removed under heat. High vacuum was then applied affording the distillation of around 7g of 1,3-Benzodioxole and leaving a quantity
of brown-to-red liquid that failed to yield a distillate at conditions well in excess of those stated in both patents (228°c at 1mmHg). At this stage
it was desired to attempt to extract any crude product that may be present in the previous leftover residue, which was dissolved in the minimum
quantity of acetone yielding a dark brown solution which was then added to water to precipitate out a minute quantity of grey crystalline material.
I find the procedures outlined in the aforementioned patents to be lacking in some important information and I was wondering if anybody may be able to
shed some light on the issues? Any suggestions regarding the synthesis and/or workup are more than welcome and I will do my best to attempt reasonable
suggestions and report back here.
Feel free to openly discuss or ask anything regarding this interesting compound here in this thread.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Well, I miss the key information: Did the reaction even occur? What does TLC reaction monitoring say?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
'Black rubbery solids' 
|
|
|
spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
Hello again and thankyou for your replies.
I must say that the appearance of these did concern me hugely, these solids are marginally soluble in water and highly soluble in Acetone and firstly
the substance took on the properties of a thick oil before becoming rubbery and solid after the addition of the DCM and water.
Unfortunately I do not currently have access to suitable TLC plates at this time as the cheap ones provided are old and aluminium-backed and so just
crack and break up immediately when cut down and so have been abandoned. I am hoping to be able to get hold of glass-backed plates however apparently
they are four times the price. I do not have access to a pure product and so whatever reaction is taking place (indicated by the colour change, change
in phase of constituents, deficit in recovered starting material) I have no way of confirming if it is yielding the desired product (without isolating
it, of course).
[Edited on 17-7-2013 by spicemaster]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
To me it sounds like your methylenedioxbenzene is breaking down and making black glop.
I would try runiing the reaction at say 100, 125 or 150 C.
1,3-Benzodioxole boils at 173 C and I think that is just too hot.
|
|
|
spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ScienceSquirrel  | To me it sounds like your methylenedioxbenzene is breaking down and making black glop.
I would try runiing the reaction at say 100, 125 or 150 C.
1,3-Benzodioxole boils at 173 C and I think that is just too hot. |
Hello again thanks for your interest. I would have assumed it was degradation or polymerisation however in Shulgin's patent relating to the successful
procedure he states: "The crude reaction mixture was freed of all volatiles ... yielding 42g of a heavy black oil." which along with the expected loss
of vapour colour in the reaction mixture (as noted in the 1954 paper relating to successful acylations) may indicate that the reaction itself is
taking place and that it is either being degraded in the final half hour when the black solids appear (if the vapour is acting as an important heat
sink transferring energy in to the condenser and the mixture itself is overheating) or that the error lies in the workup procedure. I would be happy
to explore other extraction techniques, it is unfortunate that a Bisulfite extraction is unlikely to work with this ketone due to steric hindrance.
|
|
|
spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
Upon further experimentation it would seem that the desired product is not being produced or is being destroyed at some point in the process as
further attempts at extracting any significant organic soluble material came to nothing on removal of solvent, any suggestions would be greatly
appreciated.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
Most of this is way over my head, but this caught my eye:
Quote: Originally posted by spicemaster  | | This solid was broken up and stirred under 200ml of DCM and 200ml of distilled water and the organic fraction separated and washed with dilute Sodium
Bisulfite solution. The DCM was removed by heating |
Wouldn't this form the bisulfite adduct of DCM?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
And what is a "bisulfite adduct of DCM" supposed to be? Why would dichloromethane form an adduct with bisulfite?
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
And what is a "bisulfite adduct of DCM" supposed to be? Why would dichloromethane form an adduct with bisulfite? |
It would be me having a brainfart with acronyms(somehow connecting "DCM" with methyl ethyl ketone.)
My bad  . .
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Back in the day, I sometimes produced similar products. Your product is a high boiler and it is a bitch actually vacuum distill it. If you aren't
quite getting the level of vacuum you are shooting for, you will be disappointed.
Also the Methylenedioxy ring is somewhat strained and is subject to cleavage.
Steam distillation is a more gentle alternative.
Neutralize, and rinse the original reaction mass with H2O, discard water. Then, attempt to steam distill the oil with non-acid H2O. This involves
a large volume of distillate, but might prevent the deterioration of your product.
[Edited on 1-10-2013 by zed]
|
|
|
spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
Thankyou Zed, I have attempted your procedure which following steam distillation afforded a dark oil (that Iodine really is a bitch to get rid of!)
which would seem to be either just unreacted Benzodioxole, or (hopefully) a mixture of unreacted Benzodioxole and product. I intend on running an FTIR
of the sample in order to see if there is any Ketone peak which if present I may try to find a solvent system suitable for crystallising just the
product rather than boiling off the Benzodioxole and risk charring.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Are you using a school lab? You can get some good tlc plates on ebay, craigslist, other places for ~ $20/box. I cut these into 2" strips. I hate
black sludge. Since the ketone didn't distill it probably means something went wrong. As nicodem mentioned, you should check the reaction progress
with TLC. I wonder if Fe and propionyl Cl would be a better bet? Or propionyl Cl and I2 as per the ACS article? @Nicodem: why is I2 a good catalyst
for this? (I like the piperonal route better).
"1,3-Benzodioxole boils at 173 C and I think that is just too hot." amen. Reflux under reduced pressure and maybe N2 atmosphere.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
spicemaster
Harmless

Posts: 6
Registered: 16-7-2013
Member Is Offline
Mood: No Mood
|
|
Upon running diamond ATR spectra of a few samples, it would appear that the steam distillation product shows a spectra characteristic of
1,3-benzodioxole. I believe in my attempts at running the reaction at a temperature to avoid charring has in fact prevented the product from being
formed. That being said, an old sample that I found from a previous attempt was also analysed and provided a similar spectra along with a peak at
1734cm-1 which would be indicative of a C=O ketone stretch so it seems that this reaction can indeed be successful, the real problem is in the
effective isolation and purification due to the boiling point.
|
|
|
zephler1
Harmless

Posts: 41
Registered: 16-11-2013
Member Is Offline
Mood: No Mood
|
|
hrmm..ketone is in the wrong spot, which means it would be a pain to get it into the proper spot...possible, but a mess
|
|
|