| Pages:
1
2
3
4
5
..
77 |
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
A nice shot of a crystal of naphthalene growing on the end of a thermometer.
Cheers,
O3

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
sodium sulfate crystals, sadly they started showing white spots after a few hours
 
all above information is intellectual property of Pyro.  |
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Copper(II) acetate
6 weeks growing crystal

|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Pumping down an air sensitive ligand:

A few mg of the the same ligand coordinating iron (II) ready to go for NMR, it is hard to tell from the picture but it is very very purple.

|
|
|
thebean
Hazard to Others
  
Posts: 116
Registered: 26-9-2013
Location: Minnesota
Member Is Offline
Mood: Deprotonated
|
|
<del>Sorry about the link</del>
Erythritol recrystallization from Truvia sweetener, the black and red chunks are from an oven mitt I was wearing when dissolving the Truvia, they pose
no threat to the erythritol or ETN synthesis because they're totally insoluble. Largest crystal looked to be a little over an inch, I'll weigh them
when they're dry. Shot on an iPhone 4S but I can get better photos later.

<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: del;
replaced link with attachment]
[Edited on 12.11.13 by bfesser]
"You need a little bit of insanity to do great things."
-Henry Rollins
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Some Potassium dichromate I made from Chromium(III)oxide and Potassium nitrate. That is the unfiltered product with some unreacted oxide on the
bottom.

<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: attached
image from questionable website]
[Edited on 12.11.13 by bfesser]
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
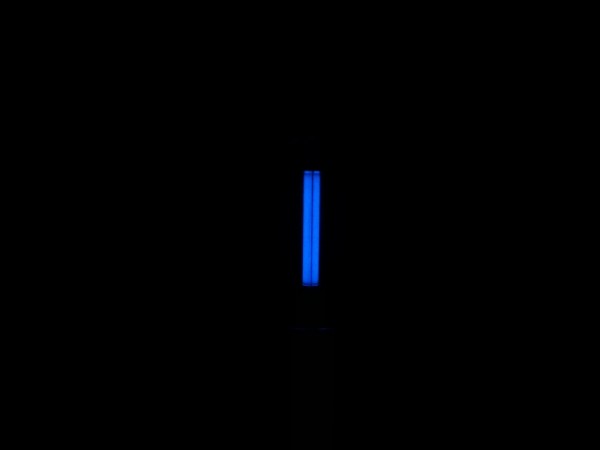
Two images from my fun times with my buddy's DSLR:
Giant images - Enjoy this link 
http://imgur.com/OdxIrgw,x3ACKvM#0
First is a 30-second exposure of Am241 modules under a silver-activated screen. Screen is about 1.5" across. 1-second light painting to pick up the
rest of the image.
Second image is a 10 second exposure of a Tritium glass vial, with the phosphor coating. Resting material is white cotton, length about 6".
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
The tritium looks cool, I always wanted to photograph something like that(:
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
In keeping with the spirit of the Tritium pictures here is my small sample, used on my periodic table display, since it is my favorite isotope of
Hydrogen.

And here is a recent element addition that's quite beautiful, a very large sample of Zirconium metal.

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
That Zirconium looks nice  Would love to have that in my collection. Would love to have that in my collection.
Some Chromium(II)chloride that has absorbed so much water that it's nearly impossible to cruch the chuncks. I think it's time for a new bottle 
But still a beautiful green when you look inside the bottle.
http://i.imgur.com/b0mkGL8.jpg
Some Dimethylglyoxime with [Ni(NH3)4]2+
The upper layer is the Ni-DADO and the purple layer below the Ammonia-Complex. ( For some reason the image is flipped, I don't know why )
http://i.imgur.com/FbJwxKn.jpg
That orange stuff is a Cobalt(III) solution. Unfortunately I forgot how I made it. ( Image flipped,too )
http://i.imgur.com/zl4TgN6.jpg
Last picture is some Bromohexane...well it was supposed to be that. I made it by reacting Bromine with Hexane. Some weeks later these black dots
appeared and formed a dark layer. It also started to smell quite stange. Must be some side product or contamination.
http://i.imgur.com/1nFtQ2U.jpg
I will upload more colored coordination compounds soon. And sorry for the image flipping. I will try to correct it.
[Edited on 13-11-2013 by fluorescence]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Terbium piece
I got 5.7 grams of terbium for a science project. I took this with my phone which has a really good macro setting.
The sample is graphite-gray and darker than I expected. Probably some oxide contamination.
Grid size is ¼" per square.
fluorescence: That chromium chloride looks appetizing...

At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
@Brain&Force: Thanks, I don't plan to taste it soon xD. I bought that a chemistry store quite cheap. The kept it in a hughe bottle and gave me the
rests of it. I could have bought a waterfree chloride but that would have costed too much. But perhaps it's time to buy a new one.
That Terbium looks oxidized. If you need it for a project why didn't you store it under inert gas oder oil ?
How much did it cost you ?
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Dopachrome
Fluorescence, terbium is not very reactive towards dry air and doesn't seem to corrode, especially not in my location's dry air. See Dave Hamric's lanthanide corrosion test. It's mostly the rare earths from lanthanum to europium that corrode quickly. Also the terbium looks dark because I
couldn't get good lighting. But it's not as bright as seen in some pictures. Terbium(III,IV) oxide is black like manganese dioxide and a little oxide
makes it turn very dark.
Terbium costs $32/5g where I got it. This is the only reasonable place to buy terbium, even for laboratories. At Sigma and other suppliers it costs about $500/1g.
And to keep with the thread, here is dopachrome formed by the addition of tyrosinase (sourced from mushrooms) to levodopa.

At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
I want that zirconium! It's wonderful! But, seriously though, rhenium, tantalum and molybdenum are so much better hahaha 
EDIT: Back to the real world, I do not own Re, Ta nor Mo... 
[Edited on 18-11-2013 by Eddygp]
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Eddygp  | I want that zirconium! It's wonderful! But, seriously though, rhenium, tantalum and molybdenum are so much better hahaha 
EDIT: Back to the real world, I do not own Re, Ta nor Mo... 
|
I might have some scrap Ta and Mo...not saying it's pretty, though.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
A few new ones from me:
Filter PPT

Freshly Filtered

Funnel Layers

Just Mixing

Unknown Crystals (Need Column to purify I think.... 

Sublimed Iodine

Schrodinger's Cat Results

Cl2 in Flask

Cl2 Apparatus

[Edited on 20-11-2013 by zenosx]
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
I Like Dots
Hazard to Self
 
Posts: 69
Registered: 10-4-2013
Member Is Offline
Mood: frisky
|
|
Just got a position in a lab at my university. I grow cells... Zombie cells! >
They are cancerous brain cells from a man.
This is Zoomed in, taken at 10x

This is a full shot of my favorite image. Well its just a thumbnail of a 2.4gb image.

|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
Wasn't sure If I had posted my rats, so here are some rats in a jar
  
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Two four week old males, are presumed +/-? Ore preserved +/-? Please explain the last bit.
<strong><a href="viewthread.php?tid=25808#pid296733">Well...</a></strong>
[Edited on 21.11.13 by bfesser]
|
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Formation of Elemental Sodium by Electrolysis Into an Incandescent Lightbulb
Not exactly original, but it's one of the most showy and blinding preparations I've seen in my time at the bench.
  
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Some vacuum distilled phenol crystals in a 50ml RBF. These were prepared by decarboxylating salicylic acid. I am working on trying to boost the yield.
It currently stands at 57%

Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
@Sublimatus - Absolutely gorgeous!
|
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Thank you. 
The lights were actually on in the room, but of course the camera tries to balance the image against the brightest source in the shot. When that's an
incandescing filament, everything else is dark. I rather liked the eery effect though, so I didn't do anything to try to fix it.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Quote: Originally posted by UnintentionalChaos  | | Some vacuum distilled phenol crystals in a 50ml RBF. These were prepared by decarboxylating salicylic acid. I am working on trying to boost the yield.
It currently stands at 57% |
Will you be making a video of this? I would really like to see it 
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


From right to left: the hydrogen comes in, bubbles through the flask and the reaction mixture, the reagent is added slowly to the mixture from the top
placed addition funnel and the hydrogen what didn’t reacts exits at the left side through a bubbler.
Sadly it overreacted even with these mild conditions and I didn’t get the product what I needed):
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
| Pages:
1
2
3
4
5
..
77 |