| Pages:
1
2 |
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
4 ways to make nitrous oxide
This page is about a method of making nitrous oxide from urea, sulfuric acid and nitric acid, and also shows 3 other ways that might not work as good:
http://www.erowid.org/archive/rhodium/chemistry/nitrous.html
A process in which ammonium nitrate is subjected to pyrolysis;
A process in which ammonia is oxidized in a gas phase in the presence of a catalyst;
A process in which sulfamic acid and nitric acid are reacted with each other; and the like.
I looked at one of the examples and it seems that it is a long-term reaction that takes a 5 hour wait, is this correct, or can I actually watch a
balloon fill up with N2O in a rapid reaction? Would the reaction of sulfamic acid and nitric acid be faster? Decomposition of ammonium nitrate is
dangerous because it can explode if it gets too hot, and I don't have a hot plate with an easily controllable temperature, just a meker burner
[Edited on 29-8-2013 by Cou]
|
|
|
MichiganMadScientist
Hazard to Self
 
Posts: 55
Registered: 22-7-2013
Member Is Offline
Mood: No Mood
|
|
Erowid? Heh..........Carefull little eyes what you see. Just saying.. 
I think the sulfamic acid/nitric acid routine would be the easiest. Drip the HNO3 onto the Sulfamic acid, and my guess is with a little heating you
will get the out-gassing of your desired product. You might also get some nasty NOx gases though, as well.....
You might want to start thinking about getting rid of the meker burner. Open flames in chemistry is really quite dangerous. I understand that you're
just a kid trying to make it in the very adult world of chemistry, and that funds for a hotplate might be nonexistant. However, I saw somewhere on
these boards where someone flipped over an eletric clothes iron and mounted it to a workbench. That might not be a bad alternative to using an open
flame. Just an idea.
AND, just in case you had some ideas regarding huffing your product. Please, Just Don't. If you need someone to explain the dangers regarding
this, you are probably too young to be making nitrous oxide. 
Also, I realize the wiki diagram below uses the ammonium nitrate route, but the same setup could be used (at least in my mind) for the sulfamic/nitric
acid route.
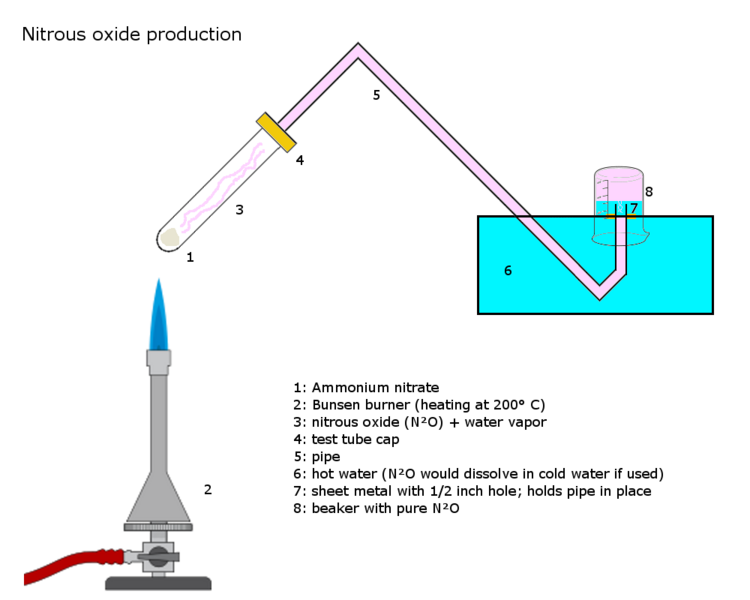
[Edited on 29-8-2013 by MichiganMadScientist]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I already know it's dangerous because it has nitrogen dioxide in it. I hope the DEA isn't reading this thread and getting the wrong idea :|
|
|
|
MichiganMadScientist
Hazard to Self
 
Posts: 55
Registered: 22-7-2013
Member Is Offline
Mood: No Mood
|
|
Its just that people tend to visit Erowid for........erm...."less-than-legitimate" reasons.....and we don't need any K3wl to google "how to make
nitrous," and find this website.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Oh shoot, I didn't realize erowid was about making drugs until I went to the main page... i thuoght it was a legit chemistry website
|
|
|
MichiganMadScientist
Hazard to Self
 
Posts: 55
Registered: 22-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Cou  | | Oh shoot, I didn't realize erowid was about making drugs until I went to the main page... i thuoght it was a legit chemistry website
|
Are you being a smart arse???    0. In case you honestly didn't know that's what Erowid is about....well, now you do. 0. In case you honestly didn't know that's what Erowid is about....well, now you do.
The scary thing is that you get dumb people that will try to make nitrous using half-baked methods, and then just assume that anything they make is
safe enough to use.
[Edited on 30-8-2013 by MichiganMadScientist]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
There are plenty of existing threads on N2O. Search on google for "sciencemadness nitrous oxide" and you may find something of assistance.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Check garagechemists thread on making nitrous oxide through the thermal decomposition of pure ammonium nitrate. He is a very skilled chemist, but
still had trouble getting rid of all the NOx. Not all is removed by even several alkaline washes, and the NO removal requires even greater lengths. If
pure, food safe nitrous is what your wantingm then I would suggest uying a pack of cartridges of it. it is used to make your own whip cream, it is
very pure, and is about $15 usd for 24 CO<sub>2</sub> like cartridges. Found online, or at your local head shop...
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
MichiganMadScientist
Hazard to Self
 
Posts: 55
Registered: 22-7-2013
Member Is Offline
Mood: No Mood
|
|
He's 14 year's old. I seriously hope we are not helping him with substance abuse. I think he is just trying to make this stuff for the hell of
it.....but you get my drift. 
[Edited on 30-8-2013 by MichiganMadScientist]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Not that i'm going to inhale it now that i found it causes nerve damage, but isn't it possible to remove the nitrogen dioxide, since it has such a
high boiling point you could just put it in the freezer and watch it condensate, then pour it out?
[Edited on 30-8-2013 by Cou]
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Relax Michigan scientist. If his goal is abusing nitrous then I doubt online lectures will prevent that, and I would rather him not inhale a lung full
of NOx if that is the case. He may need pure nitrous oxide for demos or synthesis...
Also, there are much worse things than a little laughoing gas...
edit: Coa, search for and read the thread I mentioned please.com It is very informative about purification.
[Edited on 30-8-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
They use it in the dentist as a pain blocker because it releases beta-endorphin but it's purified of nitrogen dioxide
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
With respect to "A process in which ammonium nitrate is subjected to pyrolysis", you may wish to review the discussion at http://www.sciencemadness.org/talk/viewthread.php?tid=22566&...
Also, another interesting (but untried by myself) path via Zn and very dilute HNO3 is mentioned in the thread.
Here are my entire remarks:
In my opinion, the potential significant danger of someone intentionally upon washing or accidentally inhaling the raw gas mix (as described above) is
not from solely the NO gas and its formation of NO2. The latter acid gas may quickly produce a burning sensation that may adequately forewarn one to
quickly stop inhaling.
My concern is a bit more subtle as NH3 + NO + NO2 (from O2 and NO) could form a fine smoke of NH4NO2. Source: Per Wikipedia (http://en.wikipedia.org/wiki/Ammonium_nitrite ): "Ammonium nitrite forms naturally in the air and can be prepared by the absorption of equal parts
nitrogen dioxide and nitric oxide upon aqueous ammonia.[2]". In the case of dry NH3, the reaction has been given as follows:
NO2 + NO + 2 NH3 --> NH4NO2 + H2O + N2
Of importance, Ammonium nitrite is considered 'acutely toxic' (same source) upon ingestion, which I assume includes inhalation of the dry powder.
Upon washing the NH4NO2 fine dust with aqueous Ferrous sulfate, one may simply produce a layer of the powder floating on the liquid. Bubbles of your
'pure' N2O could then be contaminated by the NH4NO2, or not, but my uncertainty suggests find another path like the previously discussed preparation
via sulfamic acid and nitric acid.
-----------------------------------------------------------
Here is an alternate preparation that suffers also from the nitrite problem as well. To quote (see http://www.transtutors.com/chemistry-homework-help/s-and-p-b... ):
" i) Very active metals such as Mn, Mg, Ca, etc. give H2 on treatment with very dilute HNO3 (2%).
(ii) Less active metals like Cu, Hg, Ag, Pb etc. give NO with dil. HNO3 . Zinc, however, gives N2O with dil HNO3 and NH4NO3 with very dilute HNO3.
Zn + 10HNO3 ( dilute ) → 4Zn(NO3)2 + N2O + 5H2O "
Now, nitrate salts in the presence of Zinc (see http://www.microbelibrary.org/library/laboratory-test/3660-n... ) and perhaps just NO as well (see http://www.cee.mtu.edu/~reh/papers/pubs/non_Honrath/fischer9... ) may also introduce some nitrites. So this preparation route does not solve the
potential nitrite contamination issue discussed above.
Also, the above reaction is not clean as varying concentrations of Nitric acid can produce varying products. For example:
Zn + 4 HNO3 (Conc) --> Zn(NO3)2 + 2 NO2 + 2 H2O
Now, for the record, I may be over stating the potential threat of Ammonium nitrite poisoning in these N2O preparations, but, at least, I won't both
be dead and wrong.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MichiganMadScientist  | You might want to start thinking about getting rid of the meker burner. Open flames in chemistry is really quite dangerous.
[Edited on 29-8-2013 by MichiganMadScientist] |
No, you don't. A Meker burner is a great thing to have and a lot of chemistry is hard to do without a strong source of heat. Open flame heat
can in any case easily be tempered by a sand bath for instance.
But it's better to ALSO have other heat sources like a decent electrical hot plate. In terms of different heat sources available for a chem lab, I'd
say: 'the more the merrier'.
[Edited on 30-8-2013 by blogfast25]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i think if we want to cut the chances alot for most of the k3wls finding this thread, you might want to correct 'nitrous oxide' to N2O instead (:
|
|
|
MichiganMadScientist
Hazard to Self
 
Posts: 55
Registered: 22-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by MichiganMadScientist  | You might want to start thinking about getting rid of the meker burner. Open flames in chemistry is really quite dangerous.
[Edited on 29-8-2013 by MichiganMadScientist] |
No, you don't. A Meker burner is a great thing to have and a lot of chemistry is hard to do without a strong source of heat. Open flame heat
can in any case easily be tempered by a sand bath for instance.
But it's better to ALSO have other heat sources like a decent electrical hot plate. In terms of different heat sources available for a chem lab, I'd
say: 'the more the merrier'.
[Edited on 30-8-2013 by blogfast25] |
I'm all for having more chemistry lab equipment than less.......but lets be honest, there very little in chemistry these days that requires an open
flame (and which can't be accomplished with using a hotplate).
I'm not saying to literally throw out the meker burner, I'm just advising that a hotplate setup ---- improvised or not ---- is going to be a bit safer
for a lot of applications. I dunno. Maybe I'm just jittery around open flames....
That's all.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, that might explain a thing or two.
Once you want to go above 300 C or so, NG/propane burners become the cheapest option. Furnaces and such like are expensive compared to Bunsen/Meker
burners and not cheap to run either.
Open flame heating is really only to be avoided where inflammable solvents/reagents that may be difficult to contain are being heated. There they are
a real no go area.
For common fusions etc good old Bunsens or even propane fired kilns/furnaces remain an excellent option. For 300 - 500 C test tube reactions that
don't involve inflammable reagents/reaction products/by-products Bunsens are also more than workable. Not to mention medium high temperature gas-solid
reactions carried out in quartz tubes.
In the case of the controlled decomposition of ammonium nitrate to N2O, direct flame tempered with a sand bath with god temperature
monitoring could work quite safely, especially carried out behind a safety screen.
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Here's where I got mine:
http://www.ebay.com/itm/48-Whipped-Cream-Chargers-Nitrous-Ox...
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
You may also enjoy inhaling diethyl ether and ethyl chloride! 
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Now I know this isnt a way to make nitrous and its not the most economically feasible but heres my method to getting some nice sweet smelling nitrous


The tank on the right is nitrous oxide contaminated with sulfur dioxide to deter inhalation and ruin your engine, luckily from my research I found a
nice method to remove this pesky SO2 by passing it through a gas washer or 2 (shown in middle) filled with a strong basic solution (usually NaHCO3 for
me) and then bam fairly pure N2O. I personally have tried the pyrolysis method and found its not very easy to collect the gas nor did I feel very safe
heating the ammonium nitrate as well as it created a nasty smoke and presumably other nitric oxides. Id stick to whippets as a source of nitrous for
the beginner as they are cheap and do not require filtering. And of course do not inhale! I only filter mine to protect my engine!
Here is a very good site (in my opinion) to read up about various sources of nitrous and how they can be obtained or made http://www.justsayn2o.com/
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I never understood how you bubble a gas through a liquid, such as bubbling hydrogen chloride through water to make hydrochloric acid. Is there an
apparatus for that?
[Edited on 31-8-2013 by Cou]
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
OK, so if you put a straw down to the bottom of a glass of water and blow into the straw, what happens. Your breath travles down the straw, and
bubbles up through the glass of water. Then exits out the top of the glass. Very simple concept.
So, lead a tube from the gas generator into the bottom of your washing solution. Let the gas bubble through the solution (use an airstone or gas
diffusor to make small bubbles and increase surface area). Have another tube sealed to the top of the contianer to catch the cleaned gas as it bubbles
out of the washing solution. Should be easy to rig up DIY.
edit: Oh, and for HCl and other gasses, make a suckback trap to prevent accidents.
You really need to <b>UTFSE</b> my friend. all this has been covered and discussed to death.
[Edited on 31-8-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Heres an old diagram I made when I originally designed my setup.
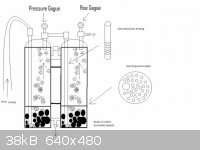
A lot of the stuff in there is optional all you really need is the tubes and the vessels filled with solution although a gas dispersion ending greatly
increases the effectivness. A common example of a gas dispersion ending would be an aquarium bubbler. I suggest you watch NurdRages youtube video on
sulfuric acid production for a good example of use of a gas bubbler.
As botonist pointed out you should really invest your time in researching things first. Incase you didnt know UTFSE stands for use the f***ing search
engine. Your post on the production of Cl2 gas from NaOCl and HCl is a painful example of your lack of researching :| But your head is in the right
place just get ideas and follow them but try to use google before you post as it saves everyone a lot of time! I personally always google whatever it
is im thinking about posting + science madness before I post a new thread to make sure that it hasnt already been discussed.
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Or it can be "use the forum search engine." Either way, points the same. Thanks for the diagram peewee, and I like your project. I was unfamiliar with
SO<sub>2</sub> being used as a deterrent in engine N<sub>2</sub>O.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
No problem sir, glad you found it interesting. Heres a closeup of the warning label. It states that ~100ppm or 0.01% SO2 has been siphoned from a
seperate tank.

When I originally was researching the project some sources said that it was adulterated with H2S which is extremely toxic however I have never seen
this actually done but conveniently enough it would be filtered the exact same way 
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
| Pages:
1
2 |