| Pages:
1
2
3
..
104 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
The Short Questions Thread (4)
In <strong>Nicodem's</strong> words: | Quote: | The <a href="viewthread.php?tid=14239">old thread</a> has become too long so please continue here. Those who never received a proper reply
in the old thread, please feel free to repost the question.
| Quote: | This is a thread where you can post all those short questions you always wanted to ask but did not consider them worthy of a new thread. You can ask
amateur science related questions of all kind as long as you think they are simple enough to be answered by other forum members in a preferably single
post.
Consequently, self discipline in avoiding off topic replies is expected. |
|
I would like to add that it would be helpful if you enter "Question"/"Answer" or something similar into the Subject field when posting.
[Edited on 7/12/13 by bfesser]
|
|
|
Variscite
Hazard to Self
 
Posts: 69
Registered: 21-5-2013
Member Is Offline
Mood: diffusing
|
|
Question
I bought some Clean Shot H2SO4 drain cleaner the other day, it says it contains inhibitors in it. It has a clear purple color to it. I was wondering
if anyone knew what these inhibitors were? Ive done some research on this, but havent found much, some say they are flourinated. I would also like to
know the purity of this stuff, ive read about suspected Se impurities (eek)! What could the applications and constraints be of this H2SO4 vs say
technical grade?
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: added to
subject field]
[Edited on 7/12/13 by bfesser]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Question
How fast does urea hydrolize in Ca(OH)2? I don't care if ammonia is still in solution I just don't want any urea present there.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: added to
subject field]
[Edited on 7/12/13 by bfesser]
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Quote: Originally posted by Variscite  | I bought some Clean Shot H2SO4 drain cleaner ..(cut).. What could the applications and constraints be of this H2SO4 vs say technical grade?
|
I don't know much about the 'inhibitors' but I have heard it has something to do with its reactivity.
The purple color is just dye to make it seem more dangerous to anyone who uses it. As you know H2SO4 is clear.
What is most likely is that your acid is recycled from some industrial plant, meaning it will be riddled with metal impurities and other unknowns,
possibly suspended carbon. The limitations are endless with recycled H2SO4 you don't want to get metal into your energetic
materials or it will sensitize them. Whatever you use the acid for will be much harder to get 'pure'.
You should be able to clear it up a little bit by heating on a hot plate (may get rid of water also) but that is very dangerous so wear eye protection
and use fume hood. Also, small additions of H2O2 should work as well look into 'Piranha solution' before attempting though.
You should also perform a titration on the acid to get its actual %, it would be nice if you could measure ppm somehow.
|
|
|
bfesser
|
Thread Split
13-7-2013 at 07:10 |
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Question
If anyone has some certified pure, finely ground anhydrous sodium bromide, could you tell me if it tends to clump or stick together, but then breaks
down easily when knocking the bottle?
I'm curious to know if the cohesion I'm seeing is due to moisture or just ionic attraction (I've ground and heated the material to 200 C, so I should
think all water has been driven off).
Thank you.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2748
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I know that dry KBr clumps readily due to moisture. But for very dry powders, often static will cause them to hold together in clumps or staticy
strings, for lack of a better word, even after they are ground into a fine powder.
|
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Answer (kind of, and to my own question)
Thanks for the input.
The <strike>KBr</strike> NaBr was finely ground after being baked for two hours at 200 C, and then loaded into a glass bottle, which was
again heated at 200 C for an hour before removing it from the oven and capping it tightly.
The <strike>KBr</strike> NaBr clumps and reclumps readily, but breaks apart relatively easily when the bottle is knocked. As two hours at
200 C should be long enough to destroy the dihydrate (which melts at 36 C) and completely dry the powder, I'm leaning towards the explanation being
ionic attraction or static as you suggest. Also the fact that sodium chloride is sold with anti-clumping and other agents to keep it free flowing
leads me to think that this is typical behavior.
Edit: I meant sodium bromide, but wrote potassium bromide. Must've had KBr on the brain after Dr. Bob referred to it.
[Edited on 7/15/2013 by Sublimatus]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
[question(s)]
<em>Q.</em> Why do hotplate manuals say not to cover the surface with Al foil?
<em>Q.</em> I plan to heat asphalt in a soup can on my metal-top hotplate. Will it be okay to put some Al foil on the top just this once?
<em>Q.</em> Should I go buy a fire extinguisher and burn cream, or just pre-dial 911?
[Edited on 27.7.13 by bfesser]
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
Aluminum is amphoteric, dissolving in either acids or bases; so beware the pH of what you're cooking.
[Edited on 2013-7-27 by ElectroWin]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Why it is prohibited to extinguish a man on fire with the help of fire extinguisher ?
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
It depends on the type of extinguisher that you use. CO2 extinguishers are highly compressed, so when they come out they're very cold, which can cause
frostbite. Also, if the person is on the ground, the CO2 can suffocate them. Some ABC dry chemical extinguishers are safe because their contents are
of negligible toxicity, but you should check their MSDS to make sure. Of course, the person will want to cover their face with their hands if you use
a dry chemical extinguisher on them, but other than that it's fairly safe.
http://www.homeofpoi.com/lessons_all/teach/Fire-Extinguisher...
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | <em>Q.</em> Why do hotplate manuals say not to cover the surface with Al foil?
[Edited on 27.7.13 by bfesser] |
I think it may be due to the aluminum reflecting the heat back to the heating surface which may cause problems with the thermostat... My ceramic top
plate also says to not heat metal vessels on it, I went against this and heated a stainless steel bowl for a hot water bath. The entire unit became
very hot after about an hour- this never happened before when using glass water baths. I could have seen it leading to a failure of some sort if I
kept it up. My theory about the aluminum reflecting the heat back may be wrong though- maybe somebody knows more on this.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Al foil can trap air pockets which insulate the heating surface, causing the aluminium plate to melt. Peach experienced this first hand IIRC... came
back to look at his reaction and found his hotplate had turned to a puddle of molten Al (exaggeration on my part, but it was pretty serious!).
|
|
|
LiD
Harmless

Posts: 13
Registered: 12-5-2013
Member Is Offline
Mood: No Mood
|
|
I would like to ask, that if I would react an alkene with OsO4 in methanol, some potassium methoxide and NMO as an oxidant, than what would be my
product be?
Or asking in another way: if I use an alcohol instead of water for opening the osmium-alkene intermedier, than would an ether form or not?
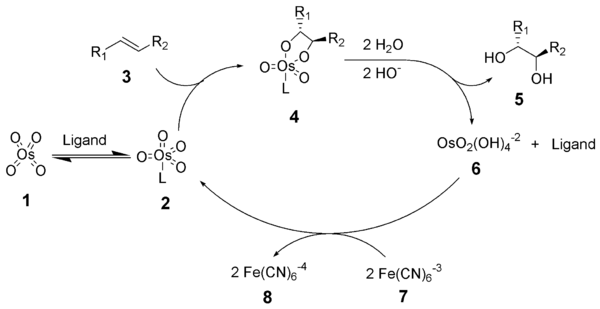
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I'm attempting a hydrogenation reaction and it calls for rhodium catalyst on alumina... I only have a small quantity of rhodium powder, can this be
used instead or is it imperative that the rhodium/alumina be used? It is for the synthesis of zingerone btw.
Thanks
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
The surface of the Rh powder, compared to the Al2O3/Rh is several fold smaller and for heterogenous catalysis this one of the most important thing.
Make some Rh/Al2O3 or Rh/C from the powder, it's not that hard to make and if you regenerate the catalyst properly, it could be used several times.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Ah, makes sense. Do you happen to have any facile ways of preparing a rhodium catalyst on alumina or carbon? I'm having a bit of difficulty locating
such a procedure...
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
From US5597772
Rhodium was next added to the fumace-aged alumina. A
14 g sample of furnace-aged alumina was mixed with an
aqueous solution of rhodium nitrate con-
taining a total of 0.085 grams of rhodium. The mixture was
first dried at room temperature, then at 100° C., and subse-
quently calcined at 550° C. for 4 hours. The process outlined
above constitutes the rhodium impregnation/drying/calcin-
ing step according to Scheme l of FIG. 2 (comparative
example). The catalyst at this point was in the so-called
“fresh” state, designated D-l , with a Rh loading of 0.6 wt %,
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kristofvagyok  | From US5597772
Rhodium was next added to the fumace-aged alumina. A
14 g sample of furnace-aged alumina was mixed with an
aqueous solution of rhodium nitrate con-
taining a total of 0.085 grams of rhodium. The mixture was
first dried at room temperature, then at 100° C., and subse-
quently calcined at 550° C. for 4 hours. The process outlined
above constitutes the rhodium impregnation/drying/calcin-
ing step according to Scheme l of FIG. 2 (comparative
example). The catalyst at this point was in the so-called
“fresh” state, designated D-l , with a Rh loading of 0.6 wt %, |
Thank you! It sounds like a bit of a challenge but ill give it a shot.
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Absorption of Water and HCl by Strong Bases
My gas generator releases small amounts of HCl gas when it's producing CO2. Additionally, some water vapor is also released. I need a
source of clean CO2 for experiments, and I was wondering what the best way tould be to remove these gases. Could I run the output through a
tube of sodium hydroxide?
Any help is appreciated.
Fear is what you get when caution wasn't enough.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
You just answered your own question. You'll lose a little CO<sub>2</sub> in the formation of
Na<sub>2</sub>CO<sub>3</sub> and NaHCO<sub>3</sub>, however. | Quote: | <table><tr><td><div align="center">13. GAS GENERATORS</div>
(a) <em>Carbon Dioxide, Hydrogen, an Hydrogen Sulphide.</em> The simplest form of generator for these gases is shown in Fig. 7. The
solid material, cracked marble for carbon dioxide, feathered zinc for hydrogen, and ferrous sulphide for hydrogen sulphide is placed in the 300-cc.
thick-walled generator bottle. The tubes are fitted as shown, and in the drying tube is placed a plug of cotton wool to strain the acid spray out of
the gas, or if the gas is to be dried, granulated calcium chloride held in place with a plug of cotton wool on either side. Enough water is poured in
through the thistle tube to cover its lower end and then about 5 cc. of 6 <em>N</em> HCl. The gas begins to generate rather slowly, but
if one is impatient and adds more acid at once the reaction will soon become so violent as to drive foam out through the delivery tube. After a few
minutes add more acid, 1 cc. at a time, in order to keep up the evolution of gas at the desired rate.<br /><br />–
<em>Synthetic Inorganic Chemistry: a Course of Laboratory and Classroom Study for First Year College Students</em>. Blanchard, Phelan,
& Davis. 5th ed., Wiley & Sons, 1936.</td><td>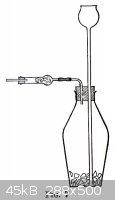 </td></tr></table> </td></tr></table> |
Oh, for fuck's sake! I just realized that we have <a
href="http://www.sciencemadness.org/library/books/synthetic_inorganic_chemistry_blanchard_5thed1937.pdf#page=28">this book in the library</a>
<img src="../scipics/_pdf.png" />—pages 18–19 in the book (28–29 in the PDF). PDFs of this and other editions are
also freely available elsewhere. I need to get used to finding things online rather than immediately jumping to my bookshelves. I'd rather not think
about how many times I may have done this in the past. This damned newfangled internets thing! After all that effort of selecting the book from my
shelf, finding the section from memory, transcribing the text, taking the photo, carefully cleaning it up in Photoshop... 
[Edited on 2.8.13 by bfesser]
|
|
|
bfesser
|
Threads Merged
2-8-2013 at 10:18 |
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Thank you.
Fear is what you get when caution wasn't enough.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Can 1,4-dimethoxybenzene be alkylated with ethylene epoxide using Iron(III) chloride?
"Imagination is more important than knowledge" ~Einstein
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Grignard Addition to Nitriles
When a Grignard reagent reacts with a nitrile, an imine salt is formed, which is usually hydrolyzed to a ketone.
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch20/ch20-3...
Is there any way to form an amine from this intermediate imine salt, without going through the ketone?
(For example, sec-butylamine from ethylmagnesium bromide and acetonitrile.)
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Yes, and in good yields. One may add diborane/THF or methanol/NaBH4 etc. For an example of the former: http://www.sciencedirect.com/science/article/pii/S0040403902...
Search on google scholar; there are plenty of examples and links to freely-accessible papers.
|
|
|
| Pages:
1
2
3
..
104 |